
Class 12 Chemistry Unit 7: Alcohols, Phenols & Ethers | Notes, Mechanisms & Reaction Maps (2025-26)
Organic compounds containing Carbon-Oxygen bonds form a massive part of industrial and biological chemistry. Unit 7 focuses on three specific classes: Alcohols, Phenols, and Ethers.
This guide breaks down their structural differences, nomenclature rules, and preparation methods, such as Hydroboration-Oxidation and the Cumene process. You will study specific reaction mechanisms, including the acid-catalyzed hydration of ethene, and learn to distinguish between primary, secondary, and tertiary alcohols using the Lucas Test.
Try Practice Questions and Answers with Hints
From understanding the acidity of phenols to mastering the cleavage of ethers with hydrogen iodide, these resources cover the essential concepts required for board exams and competitive entrance tests.
Unit 7: Alcohols, Phenols, and Ethers
Class XII Chemistry (2025-26 Session). Focusing on Competency-Based Assessment, Molecular Classification, and Reaction Mechanisms.
2025-26 Curricular Updates
The current session emphasizes competency-based education. Students must apply concepts rather than rely on rote memorization.
CBSE Focus
- Green Chemistry context.
- Mechanism identification.
- Material science applications.
ISC Focus
- Dow’s process specifics.
- Aryl ether substitution.
- Detailed mechanism coverage.
WBCHSE Focus
- Semester-based integration.
- Acidic nature of phenol.
- Traditional functional tests.
Nomenclature Pitfalls
Confusion often arises between Common and IUPAC names. Below are high-frequency exam compounds.
| Structure | Common Name | IUPAC Name |
|---|---|---|
| HO-CH2-CH2-OH | Ethylene Glycol | Ethane-1,2-diol |
| CH2(OH)-CH(OH)-CH2(OH) | Glycerol | Propane-1,2,3-triol |
| OH-C6H4-CH3 (ortho) | o-Cresol | 2-Methylphenol |
| C6H5-O-CH3 | Anisole | Methoxybenzene |
| C6H5-O-C2H5 | Phenetole | Ethoxybenzene |
Structural Geometry & Hybridization
| Molecule | Angle | Reason |
|---|---|---|
| Methanol | 108.9° | Lone pair repulsion. |
| Phenol | 109.0° | sp2 carbon effect. |
| Ether | 111.7° | Steric repulsion. |
Physical Properties
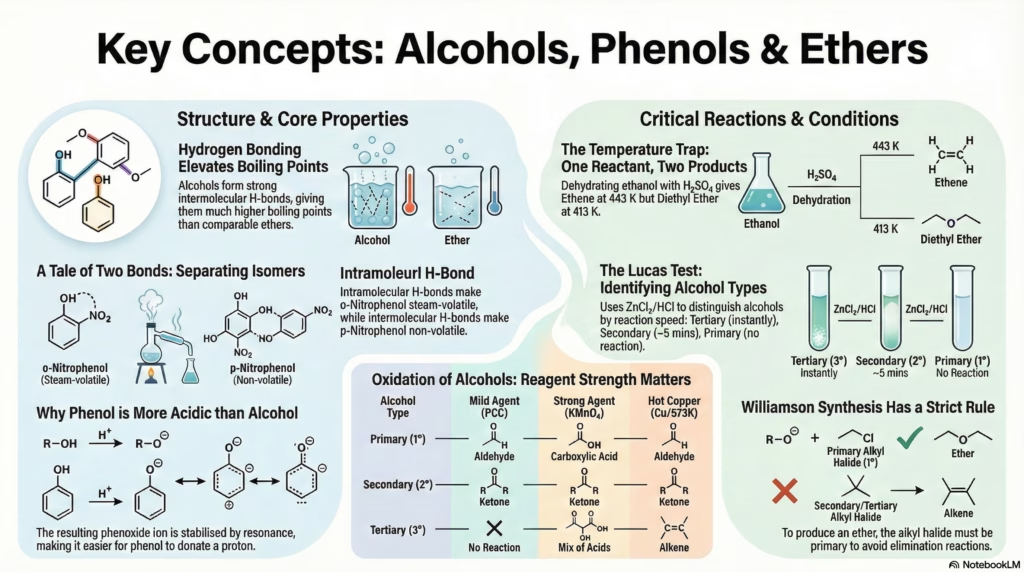
Boiling Point Trends
Alcohols have significantly higher boiling points than hydrocarbons and ethers of comparable molecular mass due to Intermolecular Hydrogen Bonding.
Solubility Dynamics
Lower Alcohols
Highly soluble in water due to H-bonds with water molecules.
Effect of Mass
Solubility decreases as the hydrophobic alkyl group size increases.
Separation: o-Nitrophenol vs p-Nitrophenol
Ortho-Nitrophenol
Volatile (Steam Distillable)
Due to Intramolecular H-Bonding (within the same molecule), it does not associate strongly with other molecules, lowering its boiling point.
Para-Nitrophenol
Non-Volatile (Remains in flask)
Due to Intermolecular H-Bonding (between different molecules), it forms associated aggregates, significantly raising the boiling point.
Mechanism Explorer
InteractiveTopic: Acid Catalysed Hydration of Ethene to Ethanol.
Step 1: Protonation
Hydronium ion transfers a proton to the double bond.
Step 2: Nucleophilic Attack
Water attacks the carbocation.
Step 3: Deprotonation
Loss of proton regenerates catalyst.
Preparation Methods
1. From Cumene (Commercial)
High YieldMost phenols produced via this method; Acetone is a valuable by-product.
2. Hydroboration-Oxidation
Alkene + (BH3)2 → Alcohol
Rule: Follows Anti-Markovnikov addition.
Commercial & Green Chemistry
Methanol
CO + 2H2 → CH3OH. Highly toxic (causes blindness).
Ethanol
Fermentation of molasses. Denatured with CuSO4/Pyridine.
Green Context
Ethanol is used as a bio-solvent replacing toxic chlorides, and as a fuel blend (Gasohol) to reduce emissions.
Reaction Mechanisms
Grignard Synthesis
| Reactant | Result |
|---|---|
| HCHO | Primary Alcohol |
| RCHO | Secondary Alcohol |
| RCOR | Tertiary Alcohol |
Williamson Ether Synthesis
R-X + R’-ONa → R-O-R’. Constraint: R-X must be Primary.
Tertiary Halide causes Elimination (Alkene) instead of Substitution.
Acidity & Modifiers
EWG (NO2): Increases Acidity (Stabilizes anion).
EDG (CH3): Decreases Acidity (Destabilizes anion).
Ether Cleavage (with HI)
Mechanism: SN2. I– attacks smaller group.
Mechanism: SN1. I– attacks Tertiary C (Stable Carbocation).
Ether Cleavage Decision Tree
Reacting R-O-R’ with HI. Where does the Iodide go?
Iodide attacks Tertiary Carbon.
Iodide attacks Smaller/Less hindered group.
The Temperature Trap: 413K vs 443K
The dehydration of alcohols with concentrated H2SO4 yields completely different products based on the temperature.
At 443 K (170°C)
C2H5OH → CH2=CH2 + H2O
Mechanism: Elimination.
Product: Alkene (Ethene).
Thermodynamically favored at higher temperatures (entropy increases).
At 413 K (140°C)
2 C2H5OH → C2H5-O-C2H5
Mechanism: Nucleophilic Substitution (SN2).
Product: Ether (Diethyl Ether).
Requires excess alcohol.
Synthesis of Aspirin (Acetylsalicylic Acid)
Produced by the acetylation of Salicylic acid using Acetic Anhydride in the presence of acid catalyst.
- Reactant: Salicylic Acid (2-Hydroxybenzoic acid)
- Reagent: Acetic Anhydride + Conc H2SO4
- Product: Aspirin (Analgesic & Antipyretic)
- By-product: Acetic Acid (Vinegar smell)
Resonance in Phenol & Phenoxide
Why is Phenol acidic and Ortho/Para directing?
1. Acidic Nature
The Phenoxide ion formed after losing H+ is resonance stabilized. The negative charge is delocalized over the benzene ring.
Ortho → Para → Ortho positions.
Comparison: Alkoxide ion (R-O–) has no resonance, making alcohols weaker acids than phenol.
2. Ortho/Para Directing
The lone pair on Oxygen is pushed into the ring (+R Effect), increasing electron density.
Ortho (2,6) and Para (4) positions.
Electrophiles (E+) attack these electron-rich positions.
Key Conversion Pathways
Convert Ethanol to Acetone (Propanone)
Convert Phenol to Aspirin
The Haloform (Iodoform) Test
Key Identification Test
Reagents: NaOH + I2 (or NaOI).
Positive Result: Formation of Yellow Precipitate (CHI3).
Condition: The molecule must contain the CH3-CH(OH)- group (methyl carbinol) or CH3-C=O group.
Oxidation Pathways
| Type | Mild (PCC) | Strong (KMnO4) | Cu / 573K |
|---|---|---|---|
| 1° | Aldehyde | Acid | Aldehyde |
| 2° | Ketone | Ketone | Ketone |
| 3° | None | Mix of Acids | Alkene |
Electrophilic Substitution
Phenol (OH is Activator)
- Br2 / CS2: Mono-substituted (o/p-Bromophenol).
- Br2 Water: Poly-substituted (2,4,6-Tribromophenol – White PPT).
- Conc. HNO3: Picric Acid (2,4,6-Trinitrophenol).
Anisole (OR is Activator)
- Friedel-Crafts: CH3Cl/AlCl3 gives o/p-Methoxy toluene.
- Nitration: Gives mixture of o/p-Nitroanisole.
Named Reactions
Reimer-Tiemann
Phenol + CHCl3 + NaOH → Salicylaldehyde.
Intermediate: Dichlorocarbene (:CCl2).
Kolbe’s Reaction
Sod. Phenoxide + CO2 → Salicylic Acid.
Phenol Conversion Roadmap
Interactive Lab Bench
Select a test and a sample to see the virtual result.
Ready
Reference Material
Distinction Table
| Test | Alcohol | Phenol |
|---|---|---|
| Litmus | Neutral | Blue → Red |
| FeCl3 | No Rxn | Violet Color |
| Br2 Water | No Rxn | White PPT |
Reagent Cheat Sheet
1° Alc → Aldehyde
Acid → Alcohol
Phenol → Benzene
Alc → Alkene
Alc → Ether
Phenol → Quinone
Flashcards
Tap to flipQuiz
1. Reagent to convert Phenol to Benzene?
2. Electrophile in Reimer-Tiemann?
3. Product of 3° Alcohol + Cu/573K?
Frequently Asked Questions
Q: Why is Phenol more acidic than Ethanol?
A: The phenoxide ion formed after losing a proton is resonance stabilized (negative charge delocalized over the benzene ring). The ethoxide ion is not stabilized and is actually destabilized by the +I effect of the ethyl group.
Q: Why do boiling points of alcohols decrease with branching?
A: Branching makes the molecule more spherical, reducing its surface area. This decreases the Van der Waals forces between molecules, lowering the boiling point.
Q: Why can’t we prepare Anisole from Bromobenzene and Sodium Methoxide?
A: Bromobenzene (Aryl Halide) has a partial double bond character in the C-Br bond due to resonance, making it extremely unreactive towards nucleophilic substitution. The correct method uses Sodium Phenoxide and Methyl Bromide.
Q: Why is sulphuric acid used in esterification?
A: It acts as a dehydrating agent to remove the water formed, shifting the equilibrium to the right (forward direction) to yield more ester, and also acts as a catalyst.
Q: How to distinguish between 1°, 2°, and 3° alcohols?
A: Use the Lucas Test (ZnCl2 + HCl). 3° alcohols react immediately (turbidity), 2° react in ~5 mins, and 1° do not react at room temperature.
Summary & Conclusion
This unit has covered the critical aspects of Alcohols, Phenols, and Ethers, moving from basic nomenclature to complex reaction mechanisms.
Key Takeaways:
- Structure Dictates Property: Hydrogen bonding elevates boiling points and solubility.
- Resonance Rules: The acidity of phenol and its electrophilic substitution patterns are driven by resonance.
- Reagent Specificity: The outcome of reactions (like Oxidation or Dehydration) depends heavily on the specific reagent and conditions (Temperature, Strength).
Mastering these concepts requires understanding the “Why” behind the reactions—steric hindrance, electronic effects, and stability of intermediates—rather than just memorizing equations.




