Class 12 Chemistry Unit 1: Solutions – Notes, Formulas & Visuals (2025-26)
Unit 1 of Physical Chemistry sets the mathematical groundwork for the Class 12 syllabus. This guide breaks down the behavior of homogeneous mixtures, focusing on how solutes alter the physical properties of solvents. From measuring concentration using Molarity and Molality to predicting Vapour Pressure changes with Raoult’s Law, we connect theoretical formulas with practical simulations.
Try our Practice Questions and Answers
Students often struggle with the Van’t Hoff factor and the thermodynamic logic behind Azeotropes. This module addresses those specific challenges through interactive charts and real-time calculators. Whether preparing for CBSE board exams or competitive tests, this resource aligns strictly with the 2025-26 academic curriculum, offering clear explanations of Henry’s Law, ideal vs. non-ideal solutions, and the four key colligative properties.
Unit 1: Solutions
Solutions: The Complete Guide
A resource for the 2025-26 CBSE Syllabus. Master concentration terms, vapour pressure laws, azeotropes, and colligative properties with interactive tools.
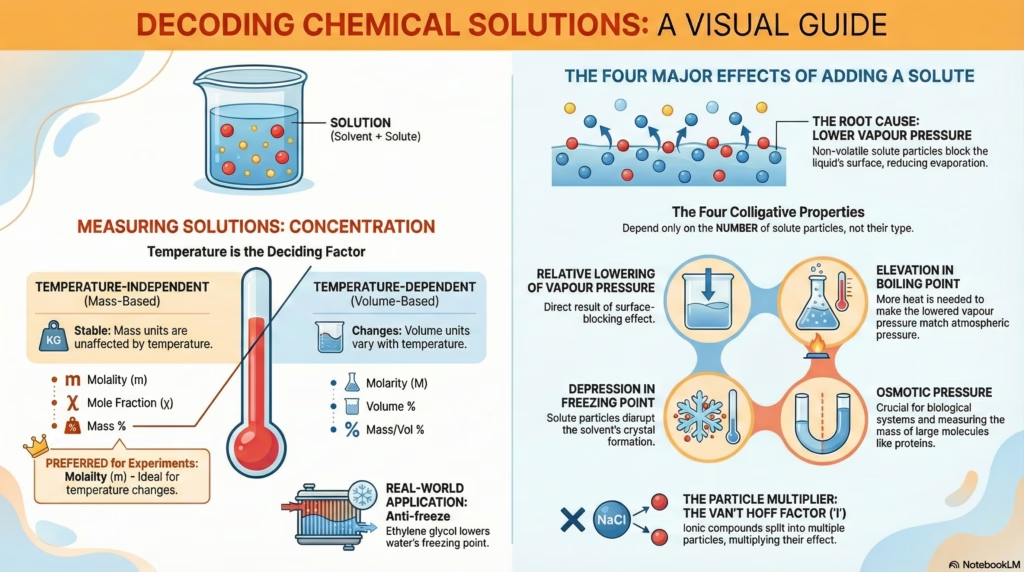
1. Measuring Concentration
Understanding solution chemistry starts with quantification. A primary distinction exists between units that change with temperature and those that remain constant. Mass does not fluctuate with thermal changes. Volume does.
Temperature Independent (Mass-based)
- Molality (m): Moles solute / kg solvent.
Essential for boiling/freezing points. - Mole Fraction (χ): Ratio of moles.
Essential for vapour pressure calculations. - Mass % (w/w): Mass solute / Total mass.
- ppm: Parts per million (Pollution analysis).
Temperature Dependent (Volume-based)
- Molarity (M): Moles solute / Litre solution.
- Volume % (V/V): Liquid in liquid mixtures (e.g. Alcohol in water).
- Mass/Vol % (w/V): Pharmacy standard (e.g. Saline drips).
Insight: As temperature rises, liquid expands (Volume ↑), so Molarity (n/V) decreases.
2. Solubility Dynamics
Solubility is not static. It depends heavily on the nature of the solute, temperature, and pressure.
🍬 Solid in Liquid
Like sugar in tea.
🥤 Gas in Liquid
Like CO₂ in soda.
Henry’s Law Visualizer
Formula: p = KH · χ
Higher KH means lower solubility at the same pressure. KH increases with temperature, which is why aquatic species are more comfortable in cold water (more oxygen).
2.1 The Surface Barrier Effect
Why does adding a solid solute lower the Vapour Pressure?
Evaporation is a surface phenomenon. Non-volatile solute particles occupy surface area, reducing the number of solvent molecules that can escape.
Blue Dots: Volatile Solvent
Gray Dots: Non-volatile Solute (Blocker)
Arrows: Vapour Pressure
3. Raoult’s Law & Deviations
| Type | Interaction | ΔHmix | ΔVmix | Example |
|---|---|---|---|---|
| Ideal | A-B ≈ A-A | Zero | Zero | Benzene + Toluene |
| Positive Dev. | A-B < A-A | +ve (Absorbs Heat) | +ve (Expands) | Ethanol + Acetone |
| Negative Dev. | A-B > A-A | -ve (Releases Heat) | -ve (Shrinks) | Chloroform + Acetone |
3.1 Azeotropes
Binary mixtures having the same composition in liquid and vapour phase and boil at a constant temperature. Fractional distillation cannot separate them.
Minimum Boiling Azeotrope
Occurs in solutions showing Large Positive Deviation. The mixture boils at a temperature lower than either component.
Maximum Boiling Azeotrope
Occurs in solutions showing Large Negative Deviation. The mixture boils at a temperature higher than either component.
4. Colligative Properties
Properties that depend only on the number of solute particles, not their identity.
1. Relative Lowering of Vapour Pressure
2. Elevation in Boiling Point
Solute lowers VP -> Higher Temp needed to boil.
3. Depression in Freezing Point
Used in car radiators (Anti-freeze).
4. Osmotic Pressure (π)
Best for calculating molar mass of proteins.
Reference: Common Constants
| Solvent | BP (°C) | Kb | FP (°C) | Kf |
|---|---|---|---|---|
| Water | 100 | 0.52 | 0 | 1.86 |
| Benzene | 80.1 | 2.53 | 5.5 | 5.12 |
⚡ Interactive: Osmosis Lab
SimulationRed blood cells behave differently depending on the concentration of the salt solution surrounding them. Select a solution type to observe the effect.
📊 Interactive: Visualizing the ‘i’ Factor Effect
Comparing the Elevation in Boiling Point ($\Delta T_b$) for different solutes at the same Molality (m). Observe how the number of particles (i) multiplies the effect.
Since $K_b$ is constant (Water), the height of the bar depends entirely on the product of $i$ and $m$.
(i=1)
(i=2)
(i=4)
🧮 Calculator: Degree of Dissociation (α)
Calculate the relationship between the Van’t Hoff factor (i) and the Degree of Dissociation (α) for electrolytes.
Formula: α = (i – 1) / (n – 1)
⚠️ Common Exam Pitfalls
Temperature Units
In Osmotic Pressure (π = CRT), Temperature (T) must ALWAYS be in Kelvin.
Mistake: Using Celsius directly.
Boiling Point vs Change
The formula ΔTb = Kbm gives the change, not the new boiling point.
Correct: Tsolution = T°solvent + ΔTb
Van’t Hoff Factor omission
Forgetting ‘i’ for ionic compounds (NaCl, K2SO4) is the #1 error.
Rule: If it’s a salt, acid, or base, check ‘i’.
Student Q&A Deep Dive
Q1: Why is Molality (m) preferred over Molarity (M) for calculating Elevation in Boiling Point?
Q2: Why do deep-sea divers use Helium in their air tanks instead of pure Nitrogen?
Q3: Why does a mixture of Ethanol and Acetone show positive deviation from Raoult’s Law?
Q4: Ethanoic acid has a molar mass of 60 g/mol. However, when determined by cryoscopy in benzene, the value approaches 120 g/mol. Explain.
Comprehensive FAQs
Why is Ethylene Glycol added to car radiators?
Ethylene glycol acts as an anti-freeze. It lowers the freezing point of water, preventing the coolant from freezing in winter. Interestingly, it also raises the boiling point, preventing the engine from overheating in summer.
Why can’t Azeotropes be separated by Fractional Distillation?
Fractional distillation works on the principle that different components boil at different temperatures. An azeotrope behaves like a pure liquid because the vapour phase has the exact same composition as the liquid phase. When you boil it, both components evaporate together in the same ratio, so no separation occurs.
What happens to the solubility of solids in liquids when temperature increases?
It depends on the nature of the dissolution process (Le Chatelier’s Principle). If dissolution is Endothermic (ΔsolH > 0, like Sugar), solubility increases. If dissolution is Exothermic (ΔsolH < 0, like Calcium Acetate), solubility decreases.
What material is used for the semi-permeable membrane in Reverse Osmosis?
Cellulose Acetate is commonly used. It is placed over a suitable support and allows solvent molecules (water) to pass through while blocking larger solute particles/ions.
Final Learning Checklist
Concepts Mastered
- Differentiating Molarity (Vol-dependent) vs Molality (Mass-dependent).
- Interpreting graphs for Henry’s Law and Raoult’s Law.
- Identifying Positive/Negative deviations based on interaction strength (A-B vs A-A).
- Understanding that Colligative Properties depend on particle count (i), not mass.
Critical Formulae
- P = KH · x (Henry’s Law)
- Ptotal = PA + PB (Raoult’s Law)
- ΔT = i · K · m (Elevation/Depression)
- π = i · C · R · T (Osmotic Pressure)
© 2026 Rezyo.in | Class XII Chemistry Module