Class XII Chemistry Unit 4: d and f Block Elements | Trends, Compounds & Exam Q&A (2025-26)
Transition and Inner Transition elements often present a challenge due to their variable oxidation states and irregular atomic trends. This guide targets the core concepts of Unit 4 for the 2025-26 Class XII curriculum, breaking down the 3d series, Lanthanoids, and Actinoids.
We examine the specific anomalies in melting points and ionization enthalpies, visualize the geometry of chromate and permanganate ions, and outline the reaction pathways for major industrial compounds. Use the interactive modules to track electronic configurations and predict magnetic properties for board exam preparation.
Unit 4: d- and f-Block Elements
A definitive guide to Transition and Inner Transition elements. Updated for the 2025-26 academic curriculum. Explore electronic configurations, magnetic properties, and the nuanced chemistry of Lanthanoids.
The 3d Transition Series
Select an element to inspect its electronic configuration.
Definition Check
A transition element has an incompletely filled d-subshell in its ground state or any common oxidation state.
Zn, Cd, Hg are typically excluded from “true” transition metals because they have full d10 configurations.
Atomic Radii Trends (3d Series)
Notice the initial contraction due to increased nuclear charge, followed by a plateau where electron repulsion counteracts the pull, and finally a slight expansion at Zinc.
Ionization Enthalpy
General increase across the period. Peaks occur at stable configurations (d5, d10).
Melting Point Trends
Melting points indicate the strength of metallic bonding. Stronger bonding occurs when more electrons (from both ns and (n-1)d) participate.
The Mn/Tc Anomaly
Manganese (Mn) and Technetium (Tc) show a sharp dip in melting point. This is because the stable half-filled d5 configuration holds electrons more tightly, making them less available for metallic delocalization.
Oxidation State Landscape
Transition elements exhibit variable oxidation states because both ns and (n-1)d electrons participate in bonding. The number of states increases to a maximum at Manganese, then decreases.
| Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn |
|---|---|---|---|---|---|---|---|---|---|
+7 |
|||||||||
+6 |
+6 |
+6 |
|||||||
+5 |
+5 |
+5 |
|||||||
+4 |
+4 |
+4 |
+4 |
+4 |
+4 |
+4 |
|||
+3 |
+3 |
+3 |
+3 |
+3 |
+3 |
+3 |
+3 |
||
+2 |
+2 |
+2 |
+2 |
+2 |
+2 |
+2 |
+2 |
+2 |
|
+1 |
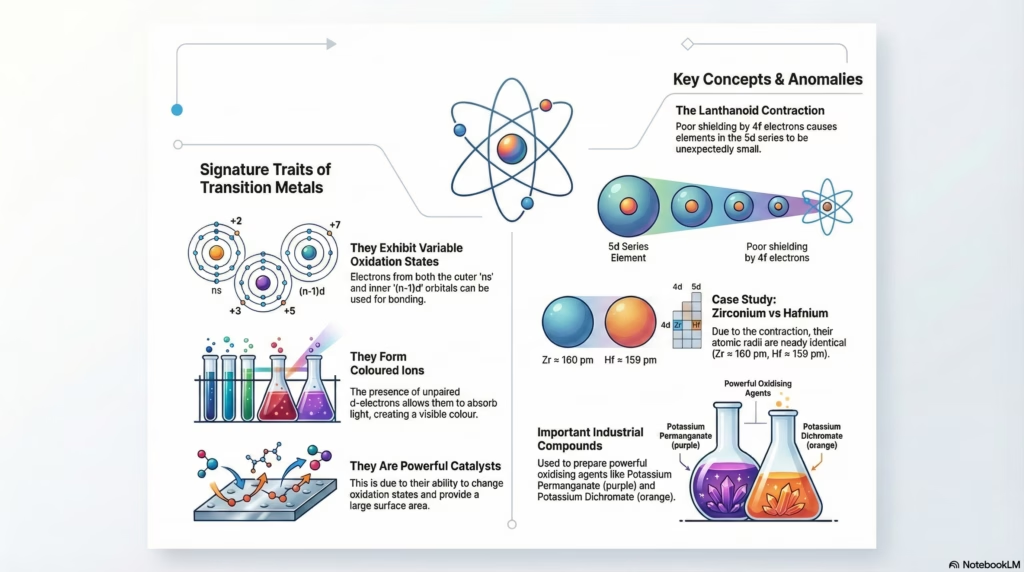
Industrial Preparation Pathways
Preparation of Potassium Dichromate
From Chromite OreFusion
Chromite ore + Na₂CO₃ + Air
(Sodium Chromate – Yellow)
Acidification
Filter & treat with H₂SO₄
(Sodium Dichromate – Orange)
Crystallization
Treat with KCl
(Crystals separate out)
Preparation of Potassium Permanganate
From PyrolusiteOxidative Fusion
MnO₂ + KOH + O₂/KNO₃
(Potassium Manganate – Green)
Electrolytic Oxidation
In Alkaline solution
(Permanganate Ion – Purple)
Reaction Profiles
Dichromate Reactions
Cr₂O₇²⁻ + 14H⁺ + 6e⁻ → 2Cr³⁺ + 7H₂O
Permanganate Reactions
Volumetric Analysis: Redox Titrations
High YieldPotassium Permanganate acts as a self-indicator in acidic medium. Here are the specific half-reactions you must know for titrations.
Green Fe²⁺ turns to Yellow Fe³⁺.
Slow reaction initially; autocatalyzed by Mn²⁺.
Forms Iodine (Purple/Brown in solution).
Sulphite oxidizes to Sulphate.
Master Equation (Acidic): MnO₄⁻ + 8H⁺ + 5e⁻ → Mn²⁺ + 4H₂O
Why do Transition Metals form Complexes?
Transition metals are unique in their ability to form a vast number of complex compounds (like [Fe(CN)₆]³⁻, [Cu(NH₃)₄]²⁺). This property drives biological systems (Hemoglobin) and industrial catalysis.
- Small Size & High Charge: High ionic charge density attracts ligands strongly.
- Availability of d-Orbitals: Empty (n-1)d orbitals of appropriate energy can accept lone pairs from ligands.
The Copper Anomaly (E° Values)
Why is E°(Cu²⁺/Cu) Positive (+0.34V)?
Unlike other 3d metals which have negative E° values (meaning they release H₂ from acids), Copper has a positive E°. This means Copper is less reactive than Hydrogen.
The stability of an ion in solution depends on the energy balance:
Energy Required (Sublimation + Ionization) vs Energy Released (Hydration).
For Copper, the high energy required to transform Cu(s) to Cu²⁺(g) is not fully compensated by its Hydration Enthalpy.
Sublimation + IE₁ + IE₂
Hydration Enthalpy
Visual representation of energy imbalance for Cu
Formation of Colored Ions
Why are some ions colored while others are white?
The d-d Transition Mechanism
In the presence of ligands (like water), the degenerate d-orbitals split into two energy levels (t2g and eg).
When white light falls on the ion, electrons absorb specific wavelengths to jump from the lower to higher d-orbital. The transmitted light is the complementary color we see.
Condition for Color:
Presence of at least one unpaired d-electron.
Ions with d0 or d10 are colorless (e.g., Sc3+, Zn2+).
Purple Solution
d-d Transition possible
Absorbs in visible region
Magnetic Moment (Spin Only):
1.73 B.M.
Structures of Oxoanions
Chromate Ion (CrO₄²⁻)
Tetrahedral Geometry
Yellow Color
Dichromate Ion (Cr₂O₇²⁻)
Two tetrahedra sharing one oxygen at 126° angle.
Orange Color
Permanganate Ion (MnO₄⁻)
Tetrahedral Geometry (π-bonding involved).
Intense Purple
Why are they Catalysts?
Reason 1: Variable Oxidation States
Transition metals can form unstable intermediates by changing oxidation states, lowering activation energy.
Ex: V₂O₅ in Contact Process (V⁵⁺ ↔ V⁴⁺)
Reason 2: Large Surface Area
Provide surface for reactants to adsorb and weaken bonds.
Ex: Finely divided Iron in Haber Process
Alloy Formation
Transition metals have very similar atomic radii (within 15%). Atoms of one metal can easily replace atoms of another in the crystal lattice.
The Lanthanoid Contraction
When moving from the 4d series (Yttrium to Cadmium) to the 5d series (Lanthanum to Mercury), we expect a size increase due to the new shell. This does not happen.
The filling of 14 electrons in the inner 4f orbitals occurs before the 5d series. 4f electrons have a diffuse shape and shield the nucleus poorly. This results in a higher effective nuclear charge pulling the outer electrons inward.
Result: The atomic radii of 4d and 5d metals are nearly identical (e.g., Zr ≈ 160 pm, Hf ≈ 159 pm). This makes separation extremely difficult.
Similarity in size between Zr and Hf is due to Lanthanoid Contraction.
The Inner Transition Matrix
Comparison between 4f (Lanthanoids) and 5f (Actinoids) series. This differentiation is critical for board exams.
High-Yield Q&A
Transition elements are defined as having incompletely filled d-orbitals in their ground state or common oxidation states.
Zn, Cd, and Hg have fully filled d-orbitals (n-1)d10 in both their ground state and stable ions (e.g., Zn2+ is 3d10). Thus, they do not fit the definition.
Scandium (Z=21) has the config [Ar] 3d1 4s2. Sc3+ loses all 3 valence electrons, resulting in [Ar] 3d0.
Since there are zero unpaired electrons, the magnetic moment is 0 B.M. It is diamagnetic.
Cr2+ to Cr3+: Cr2+ (d4) wants to lose an electron to become Cr3+ (d3). The d3 configuration is highly stable in aqueous solutions because it has a half-filled t2g level. Thus, it reduces others and oxidizes itself.
Mn3+ to Mn2+: Mn3+ (d4) wants to gain an electron to become Mn2+ (d5). The d5 configuration is a stable half-filled subshell. Thus, it oxidizes others and reduces itself.
Conceptual Deep Dive
Addressing the most common points of confusion in exams.
Why is the E° value for Mn³⁺/Mn²⁺ couple much more positive than for Cr³⁺/Cr²⁺?
A large positive E° value indicates a strong tendency to get reduced. Mn³⁺ (d⁴) is desperate to become Mn²⁺ (d⁵) because d⁵ is a half-filled, stable configuration. In contrast, Cr³⁺ (d³) is already very stable in aqueous medium (t₂g³), so it has less tendency to accept an electron compared to Mn³⁺.
Why is Cu⁺ unstable in aqueous solution?
Although Cu⁺ has a stable d¹⁰ configuration, it is unstable in water and disproportionates:
2Cu⁺(aq) → Cu²⁺(aq) + Cu(s).
This happens because the Hydration Enthalpy of Cu²⁺ is much higher (more negative) than Cu⁺ due to its smaller size and higher charge. This energy release compensates for the 2nd ionization enthalpy of Copper.
What are Interstitial Compounds?
Transition metals have crystal lattices with empty spaces (voids). Small atoms like H, C, or N get trapped here.
Key Properties:
- High melting points (higher than pure metal).
- Very hard (some approach diamond hardness).
- Retain metallic conductivity.
- Chemically inert.
Why do transition metals exhibit variable oxidation states?
This is due to the very small energy gap between the (n-1)d and ns orbitals. Electrons from both shells can participate in bonding.
However, oxidation states differ by unity (e.g., Fe²⁺, Fe³⁺) unlike non-transition elements which differ by 2 units (e.g., Pb²⁺, Pb⁴⁺) due to the inert pair effect.
Unit Summary & Outcomes
You have navigated the complexities of d and f block elements. The key takeaways for your board exams are:
Master the irregularities in Ionization Enthalpy and Atomic Radii, especially around Zn and Mn.
Always check for unpaired electrons. No unpaired e⁻ = Colorless & Diamagnetic.
KMnO₄ and K₂Cr₂O₄ are your go-to oxidizers. Remember their pH-dependent products.