Class XII Chemistry Unit 4: d & f Block Elements | Practice Q&A & Trends (2025-26)
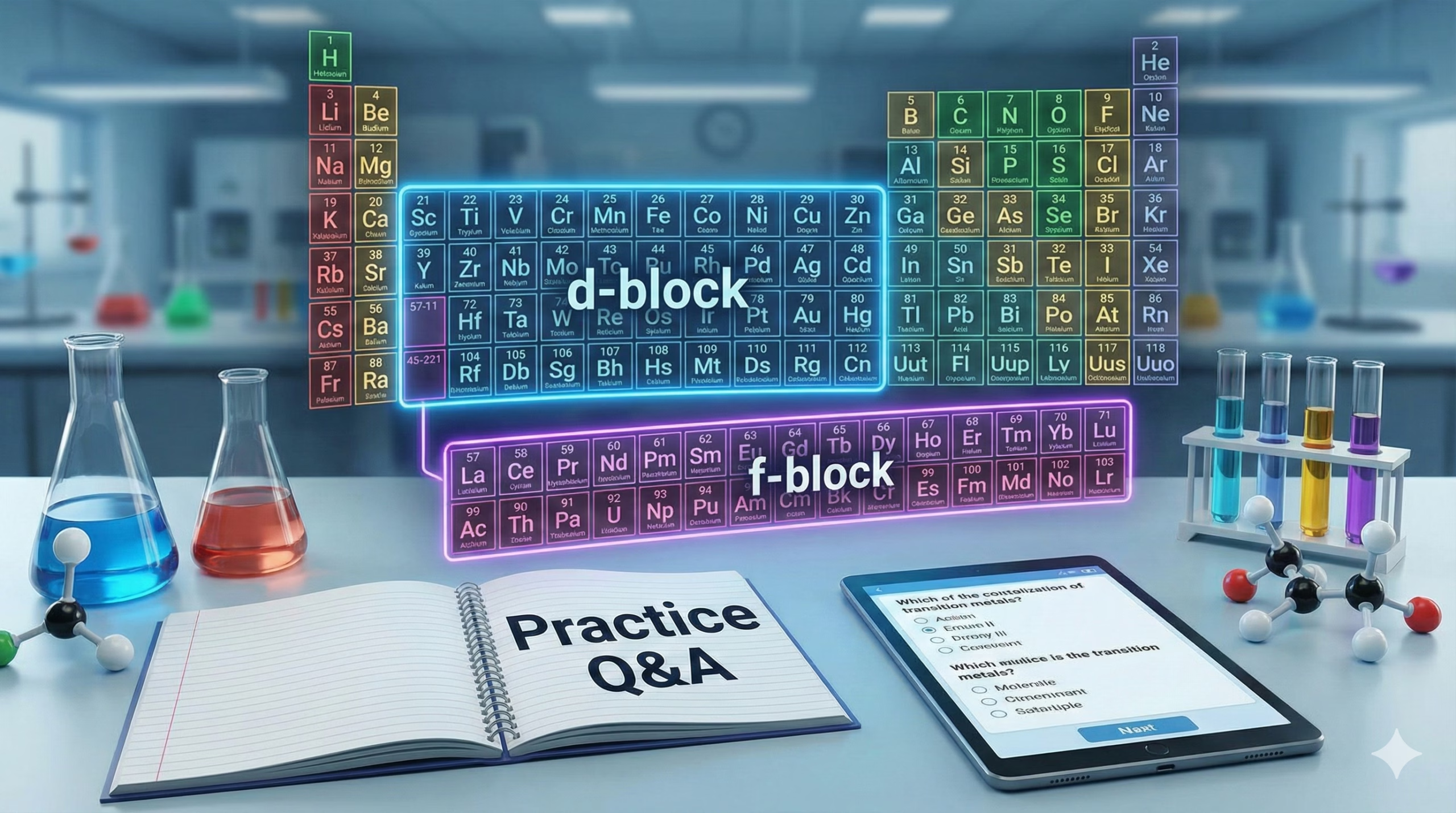
Transition and Inner Transition elements define the middle section of the periodic table, presenting a unique set of properties driven by filled and half-filled subshells. This guide breaks down Unit 4 for the 2025-26 curriculum, focusing on the electronic anomalies of the 3d series and the chemical consequences of the Lanthanoid Contraction.
We map out the trends in ionization enthalpy, visualize the geometry of chromate ions, and detail the industrial preparation of key oxidizers. Engage with the interactive modules to calculate magnetic moments and solve high-yield reasoning questions designed for board exam readiness.
Rezyo.in
Class XII Chemistry Unit 4 • 2025-26
d- and f-Block Elements Masterclass
Master the nuances of Transition and Inner Transition elements. From electronic anomalies to the Lanthanoid contraction, test your knowledge against the 2025-26 board curriculum.
Ready to Test Your Knowledge?
Enter the MCP (Master Control Panel) to attempt 25 high-yield Board Exam questions. Features live timer, instant feedback, and detailed explanations.
Spin-Only Magnetic Moment Visualizer
The magnetic character of transition metals is determined by the number of unpaired electrons (n). The formula is:
The Lanthanoid Contraction Effect
Why are Zr and Hf so hard to separate?
Normally, atomic size increases down a group. However, the 14 lanthanoid elements (filling the 4f subshell) intervene before the 5d series. The 4f electrons shield the nucleus very poorly. This results in a higher effective nuclear charge (Zeff) pulling the outer electrons in.
Despite having an extra shell, Hf is virtually the same size as Zr, leading to near-identical chemical properties.
Conceptual Deep Dive
Q: Why are Zn, Cd, and Hg not considered “Transition Elements”?
By definition, a transition element must have an incompletely filled d-subshell in its ground state or stable oxidation state. Group 12 elements (Zn, Cd, Hg) have a full d10 configuration (3d10 4s2 for Zn) in both their ground state and their common +2 ions. Thus, they lack the characteristic properties (colored ions, paramagnetism) of true transition metals.
Q: Why is Cr2+ reducing while Mn3+ is oxidizing? Both are d4.
Cr2+ acts as a reducing agent to become Cr3+ (d3), which is highly stable in aqueous solution due to the half-filled t2g level (t2g3). Conversely, Mn3+ is a strong oxidizing agent because gaining an electron converts it to Mn2+ (d5), which has a stable half-filled d-subshell.
Q: Why do transition metals exhibit variable oxidation states?
This is due to the very small energy gap between the (n-1)d and ns orbitals. Electrons from both subshells can participate in bonding. Unlike non-transition elements (where states differ by 2), transition metal oxidation states differ by 1 unit (e.g., Fe2+ and Fe3+).
Q: What happens when pH changes for Dichromate ions?
Chromates (CrO42-, yellow) and Dichromates (Cr2O72-, orange) exist in equilibrium dependent on pH. In acidic medium (low pH), the equilibrium shifts to form Dichromate (Orange). In alkaline medium (high pH), it shifts to form Chromate (Yellow).
2CrO₄²⁻ (Yellow) + 2H⁺ ⇌ Cr₂O₇²⁻ (Orange) + H₂O
Chapter Summary & Outcomes
You have navigated the architectural framework of the d- and f-block elements. From understanding the stability of half-filled subshells to the industrial applications of Potassium Permanganate, these concepts are pivotal for the board exams.