Class 12 Chemistry Unit 14: Biomolecules Notes, Structures & Interactive Quiz
Life looks complex, but chemistry breaks it down into understandable parts. Unit 14 explores the specific non-living atoms that build living organisms. From the energy stored in sugars to the genetic code in DNA, these organic compounds dictate how biology functions.
Try our Practice Exam Q&A
This guide details the structures of carbohydrates, proteins, and nucleic acids, explaining reactions like glucose hydrolysis and protein denaturation. Review the classification of vitamins, analyze the difference between DNA and RNA, and test your knowledge with the interactive quiz below.
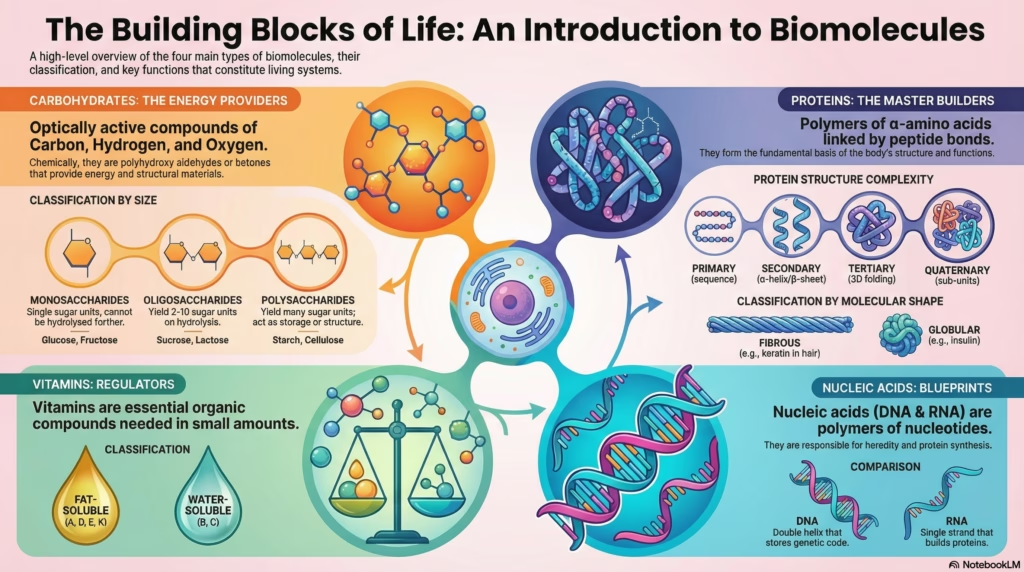
Biomolecules: The Chemistry of Life
Explore the non-living atoms that build living systems. From carbohydrates to nucleic acids, understand the molecular logic of biology.
4 Truths Hidden Inside the Molecules That Make You
Living systems grow, sustain, and reproduce themselves. The most strange fact about a living system is that it is composed of non-living atoms and molecules. We are, essentially, organized collections of chemistry obeying standard laws.
Biochemistry is the field that studies this chemistry. It reveals that the building blocks of life often follow rules that defy simple intuition. Sugars and proteins hide elegant complexities. Here are four specific details that clarify the nature of biomolecules.
1. Carbohydrates Aren’t Just “Hydrated Carbon”
The name “carbohydrate” originated from the observation that many simple sugars fit the formula Cx(H2O)y. This suggested a structure of carbon atoms combined with water molecules. Glucose (C6H12O6) fits this pattern perfectly.
This rule fails upon closer inspection. Acetic acid (CH3COOH) fits the formula but is not a carbohydrate. Rhamnose (C6H12O5) is a carbohydrate but does not fit the formula.
Correct Definition
Optically active polyhydroxy aldehydes or ketones, or compounds which produce such units on hydrolysis.
2. The “Inverting” Trick of Sucrose
Sucrose (table sugar) is dextrorotatory, meaning it rotates polarized light to the right. When hydrolyzed, it splits into glucose and fructose. The resulting mixture behaves differently. Glucose rotates light to the right (+52.5°), but fructose rotates light strongly to the left (–92.4°). The strong leftward rotation of fructose overpowers the glucose. The mixture becomes laevorotatory. This specific mixture is called Invert Sugar.
3. Cooking Only Changes Shape, Not Sequence
When you boil an egg, the white coagulates. This is denaturation. Heat disturbs the hydrogen bonds holding the protein chains in their specific 3D shape (globules and helices). The chains unfold. The biological activity is lost, but the chemical identity remains. The primary structure—the sequence of amino acids—remains intact. The blueprint survives the destruction of the building.
4. “Vitamin” is a Historical Typo
The term began as “Vitamine” (Vital + Amine) because early researchers thought all these compounds contained amino groups. Later research proved this false. The ‘e’ was dropped, leaving us with “Vitamin.” The name is a fossil of early scientific understanding.
Core Concepts & Analysis
Deep dive into structure, function, and reaction mechanisms.
Carbohydrates: Structure & Classification
Classification Logic
Carbohydrates classify based on hydrolysis products. Use the tabs below to view examples.
- Cannot be hydrolyzed further.
- Examples: Glucose, Fructose, Ribose.
- All are reducing sugars.
- Yield 2-10 monosaccharide units.
- Disaccharides: Sucrose (Glc + Fru), Maltose (Glc + Glc), Lactose (Glc + Gal).
- Sucrose is non-reducing; Maltose/Lactose are reducing.
- Yield large number of units.
- Examples: Starch, Cellulose, Glycogen.
- Non-reducing and generally tasteless.
Isomers differing only in configuration at C1 (the hemiacetal carbon). α-Glucose and β-Glucose are classic examples.
Visualizing Glucose
Canvas RenderSchematic: Fisher Projection converting to Pyranose ring
Disaccharides: The Double Sugars
Interactive TreeVisualizing breakdown products (Click nodes to expand/collapse)
| Sugar | Components | Reducing Nature |
|---|---|---|
| Sucrose | α-D-Glucose + β-D-Fructose | Non-Reducing |
| Maltose | α-D-Glucose + α-D-Glucose | Reducing |
| Lactose | β-D-Galactose + β-D-Glucose | Reducing |
Polysaccharides: Nature’s Energy Tanks
Interactive Data1. Starch Composition: Amylose vs Amylopectin
Starch is the main storage polysaccharide in plants.
Hover over segments for details
2. Glycogen (Animal Starch)
The storage polysaccharide in animal body.
3. Cellulose
Most abundant organic substance in plant kingdom.
Advanced Analysis: Synthesis & Structure
Lab Report View1. Preparation of Glucose
From Sucrose (Cane Sugar)
Process: Boiled with dilute HCl or H₂SO₄ in alcoholic solution.
Result: Equimolar mixture obtained.
From Starch
Process: Hydrolysis with dilute H₂SO₄ at 393 K under 2-3 atm pressure.
Scale: Industrial production.
2. Amino Acids: Dietary Classification
| Category | Definition | Examples |
|---|---|---|
| Essential | Cannot be synthesized by the body; must be in diet. | Valine, Leucine, Isoleucine, Lysine |
| Non-Essential | Can be synthesized by the body. | Glycine, Alanine, Glutamic Acid |
Zwitter Ion Concept
In aqueous solution, the carboxyl group loses a proton and the amino group accepts it, creating a dipolar ion.
Proteins: Architecture of Life
| Feature | Fibrous Proteins | Globular Proteins |
|---|---|---|
| Shape | Linear, fiber-like structure. | Spherical, folded structure. |
| Solubility | Insoluble in water. | Soluble in water. |
| Forces | Hydrogen & Disulphide bonds. | Weak intermolecular forces. |
| Examples | Keratin (hair), Myosin (muscle). | Insulin, Albumins. |
Protein Structure: Levels of Complexity
D3 HierarchyMapping forces and features across levels
Secondary Structure: The Two Motifs
| Property | α-Helix | β-Pleated Sheet |
|---|---|---|
| Shape | Right-handed screw (Coil) | Extended, flat sheet |
| H-Bonds | Intramolecular (Within chain) | Intermolecular (Between chains) |
Visualizing α-Helix
Schematic representation
Enzymes: Biological Catalysts
Enzymes differ from standard inorganic catalysts. They are highly specific, work under mild temperature/pH conditions, and regulate metabolic pathways efficiently.
Key Characteristics
- Specificity: One enzyme catalyzes one specific reaction (e.g., Urease only hydrolyzes urea).
- Efficiency: Can increase reaction rates by factors of 1020.
- Optimum Conditions: Function best at specific pH (usually 5-7) and Temperature (37°C).
Mechanism (Lock & Key)
1. Enzyme (E) binds Substrate (S) → [E-S] Complex.
2. Product formation → [E-P] Complex.
3. Release → Enzyme (E) + Product (P).
Vitamins & Hormones: Chemical Regulators
Vitamins: Essential Micronutrients
Stored in liver & adipose tissues. Can accumulate in the body.
Excreted in urine. Must be replenished regularly (except B12).
Hormones: Chemical Messengers
- Steroids: Estrogens, Androgens.
- Polypeptides: Insulin, Glucagon.
- Amino Acid Derivatives: Thyroxine, Adrenaline.
Essential Vitamins Checklist
| Vitamin | Common Sources | Deficiency Diseases |
|---|---|---|
| Vitamin A | Fish liver oil, carrots, butter, milk | Xerophthalmia (hardening of cornea), Night blindness |
| Vitamin B1 (Thiamine) | Yeast, milk, green vegetables, cereals | Beri beri (loss of appetite, retarded growth) |
| Vitamin B2 (Riboflavin) | Milk, egg white, liver, kidney | Cheilosis (fissuring at corners of mouth), digestive disorders |
| Vitamin B6 (Pyridoxine) | Yeast, milk, egg yolk, cereals | Convulsions |
| Vitamin B12 | Meat, fish, egg, curd | Pernicious anaemia (RBC deficiency in haemoglobin) |
| Vitamin C (Ascorbic Acid) | Citrus fruits, amla, green leafy vegetables | Scurvy (bleeding gums) |
| Vitamin D | Exposure to sunlight, fish, egg yolk | Rickets (bone deformities in children), Osteomalacia |
| Vitamin E | Vegetable oils like wheat germ oil, sunflower oil | Increased fragility of RBCs, muscular weakness |
| Vitamin K | Green leafy vegetables | Increased blood clotting time |
Nucleic Acids: The Genetic Blueprint
Interactive SunburstHover center-out to explore Base Classification
Composition Hierarchy
Phosphodiester Linkage
Nucleotides are joined by a linkage between the 5′ carbon of one pentose sugar and the 3′ carbon of the next. This forms the backbone.
Watson-Crick Model (DNA)
- Adenine (A) = Thymine (T) (2 H-bonds)
- Cytosine (C) ≡ Guanine (G) (3 H-bonds)
Interactive Mastery Quiz
Frequently Asked Questions
Why are vitamins essential if we need them in small amounts?
Vitamins act as cofactors for enzymes.
What is the difference between Glycosidic and Peptide linkages?
A Glycosidic linkage joins two monosaccharides via an oxygen atom. A Peptide linkage joins amino acids via an amide group (-CO-NH-).