
Class 12 Chemistry: Aldehydes, Ketones & Carboxylic Acids – Reactions, Mechanisms & Q&A
The chemistry of Aldehydes, Ketones, and Carboxylic Acids focuses on the carbonyl group (>C=O), the structural feature that dictates reactivity in this class of organic compounds. This guide outlines specific preparation methods, such as the Etard reaction and Grignard synthesis, while explaining the electron shifts behind nucleophilic attacks and acidity trends.
You will find detailed breakdowns of mechanism-driven processes like Esterification and Clemmensen reduction, alongside practical lab tests used to distinguish between functional groups. Use these notes to solve conversion problems and answer reasoning-based questions found in standard board examinations.
Aldehydes, Ketones, and Carboxylic Acids
This unit bridges hydrocarbon chemistry and biochemistry. The carbonyl group (>C=O) dictates the properties and reactivity. Mastery here involves understanding electron density shifts, nucleophilic attacks, and acidity trends.
The Carbonyl Center
Nomenclature & Structure
| Group | Suffix | Example |
|---|---|---|
| Aldehyde | -al | Ethanal (CH3CHO) |
| Ketone | -one | Propanone (CH3COCH3) |
| Carboxylic Acid | -oic acid | Ethanoic acid (CH3COOH) |
Visual Mechanisms & Tests
Distinguishing Tests
(Silver Mirror)
(Red Ppt)
(Yellow Ppt)
Carboxylate Resonance
Key Preparation Reactions
Rosenmund Reduction
Aldehydes OnlyConverts Acid Chlorides to Aldehydes using poisoned catalyst to prevent over-reduction.
Stephen Reduction
From NitrilesNitriles reduced to imines with SnCl2/HCl, then hydrolyzed.
Etard Reaction
Toluene → BenzaldehydeChromyl chloride oxidizes methyl group to a chromium complex, hydrolyzed to aldehyde.
Friedel-Crafts Acylation
Aromatic KetonesElectrophilic substitution on benzene ring using acyl halide.
Preparation of Carboxylic Acids
From Alkylbenzenes
Vigorous oxidation of alkylbenzenes with chromic acid or alkaline KMnO4 gives benzoic acid. The entire alkyl chain is oxidized to -COOH group regardless of length.
From Grignard Reagents
Grignard reagents react with solid Carbon Dioxide (Dry Ice) to form salts of carboxylic acids, which give corresponding acids on acidification.
From Nitriles & Amides
Nitriles are hydrolysed to amides and then to acids in the presence of H+ or OH– as catalyst.
From Esters
Acidic hydrolysis of esters gives directly carboxylic acids while basic hydrolysis gives carboxylates, which on acidification give corresponding acids.
High-Yield Study Notes
Aldol vs. Cannizzaro
The deciding factor is the α-hydrogen.
• With α-H → Aldol Condensation.
• Without α-H → Cannizzaro Reaction.
Example: Acetaldehyde (Aldol), Formaldehyde (Cannizzaro).
Acidity Trend
Electron Withdrawing Groups (EWG) increase acidity by stabilizing the carboxylate ion.
Order: CF3COOH > CCl3COOH > CH3COOH
Grignard Reagent
Always use dry ether. Any moisture will protonate the Grignard reagent (RMgX), destroying it to form a hydrocarbon (R-H).
The Ortho Effect
Ortho-substituted benzoic acids are stronger than their meta and para isomers, regardless of whether the substituent is electron-donating or withdrawing.
Properties & Solubility
Boiling Point Comparison
Compounds with similar molecular mass.
Solubility Trends
- Lower Members: Miscible in water due to Hydrogen bonding with water molecules.
- Higher Members: Solubility decreases rapidly as the hydrophobic alkyl chain length increases.
- Organic Solvents: All aldehydes and ketones are fairly soluble in organic solvents like benzene and ether.
Nucleophilic Addition Reactions
Reactivity Order: Aldehydes > Ketones
- Steric Hindrance: Ketones have two bulky alkyl groups blocking the attack.
- Electronic Effect: Alkyl groups (+I effect) reduce positive charge on Carbon.
Addition of Ammonia Derivatives
General Reaction: >C=O + H2N-Z → >C=N-Z + H2O
| Reagent (H2N-Z) | Z-Group | Product Name |
|---|---|---|
| Hydroxylamine | -OH | Oxime |
| Hydrazine | -NH2 | Hydrazone |
| Phenylhydrazine | -NHC6H5 | Phenylhydrazone |
| 2,4-DNP | -NHC6H3(NO2)2 | 2,4-DNP Derivative |
| Semicarbazide | -NHCONH2 | Semicarbazone |
Reduction & Oxidation
Clemmensen Reduction
Uses Zinc Amalgam and conc. HCl to reduce carbonyl group to -CH2-.
Wolff-Kishner Reduction
Uses Hydrazine followed by KOH/glycol heating.
Oxidation of Methyl Ketones (Haloform Reaction)
Methyl ketones react with Sodium Hypohalite (NaOX) to form a haloform (CHX3) and sodium salt of carboxylic acid. This is the basis of the Iodoform Test.
R-CO-CH3 + 3NaOX → R-COONa + CHX3 + 2NaOH
Name Reactions: α-Hydrogen
Aldol Condensation
Needs α-HTwo molecules of aldehyde/ketone condense in the presence of dilute alkali (NaOH) to form β-hydroxy aldehyde (Aldol), which dehydrates on heating to give α,β-unsaturated aldehyde.
(3-Hydroxybutanal / Aldol)
→(Heat, -H2O)→ CH3-CH=CH-CHO (But-2-enal)
Cross Aldol Condensation
Between two different aldehydes/ketones. If both have α-hydrogens, a mixture of 4 products is obtained. If only one has α-hydrogen, cross product is major.
Cannizzaro Reaction
No α-HDisproportionation reaction where one molecule is oxidized to acid salt and another reduced to alcohol. Requires concentrated alkali (50% NaOH/KOH).
(Methanol + Pot. Formate)
Carboxylic Acid Reactions
Hell-Volhard-Zelinsky (HVZ) Reaction
Specific for substituting α-hydrogen of carboxylic acids with Halogen (Cl, Br).
Used to synthesize α-amino acids.
Esterification
Reaction with alcohol in presence of conc. H2SO4.
Anhydride Formation
Dehydration using P2O5 or conc. H2SO4.
Reaction with PCl5 / SOCl2
Hydroxyl group replacement. SOCl2 is preferred as by-products are gases.
Reaction with Ammonia
Forms ammonium salt, which on heating gives amide.
Decarboxylation
Heating sodium salt with Soda Lime (NaOH + CaO).
Ring Substitution
-COOH is meta-directing and deactivating.
Uses of Common Compounds
- Methanal: Manufacture of Bakelite, urea-formaldehyde glues; 40% solution (Formalin) preserves biological specimens.
- Ethanal: Starting material for acetic acid, ethyl acetate, vinyl acetate, and drugs.
- Acetone: Common solvent for paints, nail polish remover; manufacture of polymers.
- Benzoic Acid: Food preservative (Sodium Benzoate), manufacture of dyes and perfumes.
Lab Tests & Distinctions
| Test | Reagent | Positive Result | Identifies |
|---|---|---|---|
| Tollens’ | Ammoniacal AgNO3 | Silver Mirror | All Aldehydes |
| Fehling’s | CuSO4 + Tartrate | Red Ppt | Aliphatic Aldehydes Only |
| Iodoform | I2 + NaOH | Yellow Ppt | Methyl Ketones (CH3-C=O) |
| Bicarbonate | NaHCO3 | Effervescence | Carboxylic Acids |
Exam Q&A
Q1: Why are carboxylic acids higher boiling than alcohols of comparable mass?
Q2: Distinguish chemically between Pentan-2-one and Pentan-3-one.
Pentan-2-one contains a methyl ketone group (CH3-CO-) and gives a yellow precipitate of Iodoform (CHI3) when treated with I2/NaOH.
Pentan-3-one lacks this group and gives no precipitate.
Q3: Why does benzaldehyde not undergo Aldol Condensation?
Q4: Arrange the following in increasing order of reactivity towards HCN: Acetaldehyde, Acetone, Di-tert-butyl ketone.
Reason: Steric hindrance increases from acetaldehyde (one alkyl) to acetone (two small alkyls) to di-tert-butyl ketone (two very bulky groups). Greater steric hindrance makes nucleophilic attack by CN– harder.
Q5: Explain why Chloroacetic acid is stronger than Acetic acid.
Frequently Asked Questions
Can methanal be prepared by Rosenmund reduction?
No. Formyl chloride (HCOCl), the required starting material, is unstable at room temperature.
Why are carboxylic acids stronger than phenols?
The carboxylate ion is stabilized by resonance between two equivalent electronegative oxygen atoms. The phenoxide ion disperses charge onto carbon atoms of the ring, which is less effective.
What happens when Acetone reacts with Grignard Reagent?
It yields a tertiary alcohol. The alkyl group from RMgX attacks the ketone carbon.
Is 2,4-DNP test specific for aldehydes?
No, it is a general test for the Carbonyl group. Both aldehydes and ketones give an orange/red precipitate.
What is the role of glycol in Wolff-Kishner reduction?
Glycol (Ethylene glycol) acts as a high-boiling solvent, allowing the reaction mixture to reach the temperatures required for the decomposition of the hydrazone intermediate.
Explore Other Units
Organic Chemistry
Inorganic Chemistry

Unit 14 Biomolecules Class 12 (2025-26): Practice Exam Questions
Living organisms operate through the interaction of complex organic compounds. This guide for Unit 14 covers the essential chemistry of Carbohydrates, Proteins, Nucleic Acids, and Vitamins as per the Class XII 2025-26 syllabus.
We examine how Monosaccharides link to form Polysaccharides, the specific folding patterns of Proteins, and the storage of genetic information in DNA and RNA. Use the interactive tools and practice quiz below to verify your grasp of concepts like Glucose oxidation, Peptide bond formation, and Zwitter ion characteristics.
Rezyo.in
Class XII Unit 14: Biomolecules
The Chemistry of Life
Living systems grow, sustain, and reproduce using complex organic molecules. This unit explores the structure and function of carbohydrates (energy sources), proteins (structural building blocks), nucleic acids (genetic information), and vitamins. We examine the chemical logic behind life processes, from the glycosidic linkages in sugars to the peptide bonds in proteins.
Assessment: Biomolecules
Timed challenge on Glucose reactions, DNA bases, and Protein structure.
Test Your Knowledge
Do you know the product of glucose with HI? Can you identify the non-reducing sugar? Start the quiz to verify your preparation.
Glucose Reactions
Evidence for the open-chain structure of Glucose (\(C_6H_{12}O_6\)):
- HI Heating with HI yields n-Hexane. Proves all 6 carbons are in a straight chain.
- \(Br_2\) Water Mild oxidation yields Gluconic Acid. Indicates aldehyde group.
- \(HNO_3\) Strong oxidation yields Saccharic Acid (dicarboxylic). Indicates primary alcohol.
- Acetylation Forms Glucose Pentaacetate. Confirms presence of 5 -OH groups.
Cyclic Structure
Glucose forms a six-membered ring (Pyranose). The -OH at C-5 reacts with the aldehyde group to form a hemiacetal.
Proteins & Structure
Amino Acids & Peptides
Proteins are polymers of \(\alpha\)-amino acids linked by peptide bonds (-CO-NH-).
In aqueous solution, the carboxyl group loses a proton (\(COO^-\)) and the amino group accepts a proton (\(NH_3^+\)). The molecule is electrically neutral but carries charges.
Amino acids that the body cannot synthesize (e.g., Valine, Leucine). Non-essential ones (e.g., Glycine) are synthesized by the body.
Levels of Structure
-
1
Primary
Specific sequence of amino acids. Determined by genetic information.
-
2
Secondary
Regular folding (H-bonds). \(\alpha\)-helix (right-handed screw) and \(\beta\)-pleated sheet.
-
3
Tertiary & Quaternary
Overall 3D folding (Disulphide links, van der Waals). Quaternary is the arrangement of multiple subunits.
Nucleic Acids (DNA & RNA)
DNA (Deoxyribonucleic Acid)
- Sugar: \(\beta\)-D-2-deoxyribose.
- Bases: Adenine (A), Guanine (G), Cytosine (C), Thymine (T).
- Structure: Double helix held by H-bonds (A=T, C≡G).
- Function: Genetic reserve, heredity, self-replication.
RNA (Ribonucleic Acid)
- Sugar: \(\beta\)-D-ribose.
- Bases: Adenine (A), Guanine (G), Cytosine (C), Uracil (U).
- Structure: Single strand (sometimes folds back).
- Function: Protein synthesis (mRNA, tRNA, rRNA).
Frequently Asked Questions
Why is Sucrose a non-reducing sugar?
In sucrose, the reducing groups (aldehyde of glucose and ketone of fructose) are involved in the glycosidic bond formation (C1 of Glucose and C2 of Fructose). Since there is no free reducing group, it does not reduce Tollens’ reagent or Fehling’s solution.
What happens during the denaturation of proteins?
When subjected to heat or pH change, the hydrogen bonds are disturbed. Globules unfold and helices uncoil. The protein loses its biological activity. Note: Secondary and tertiary structures are destroyed, but the primary structure (sequence of amino acids) remains intact.
What are reducing sugars?
All carbohydrates containing a free aldehyde or ketone group that can reduce Fehling’s solution and Tollens’ reagent are reducing sugars. Examples include Glucose, Fructose, Maltose, and Lactose.
Question goes here…
Explanation
Explanation goes here.
Quiz Complete!
You scored 0 out of 25.

Class 12 Chemistry Unit 14: Biomolecules Notes, Structures & Interactive Quiz
Life looks complex, but chemistry breaks it down into understandable parts. Unit 14 explores the specific non-living atoms that build living organisms. From the energy stored in sugars to the genetic code in DNA, these organic compounds dictate how biology functions.
Try our Practice Exam Q&A
This guide details the structures of carbohydrates, proteins, and nucleic acids, explaining reactions like glucose hydrolysis and protein denaturation. Review the classification of vitamins, analyze the difference between DNA and RNA, and test your knowledge with the interactive quiz below.
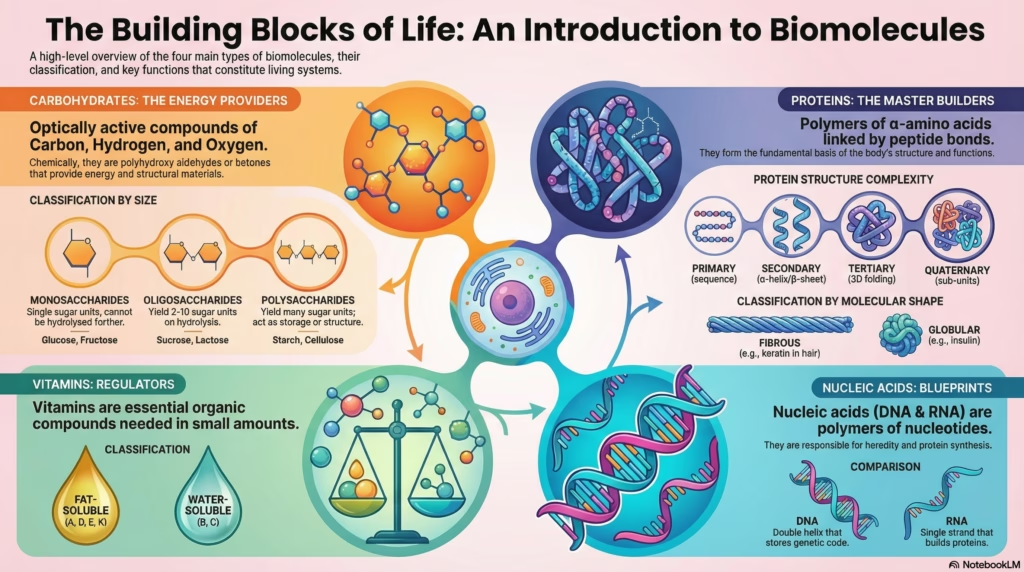
Biomolecules: The Chemistry of Life
Explore the non-living atoms that build living systems. From carbohydrates to nucleic acids, understand the molecular logic of biology.
4 Truths Hidden Inside the Molecules That Make You
Living systems grow, sustain, and reproduce themselves. The most strange fact about a living system is that it is composed of non-living atoms and molecules. We are, essentially, organized collections of chemistry obeying standard laws.
Biochemistry is the field that studies this chemistry. It reveals that the building blocks of life often follow rules that defy simple intuition. Sugars and proteins hide elegant complexities. Here are four specific details that clarify the nature of biomolecules.
1. Carbohydrates Aren’t Just “Hydrated Carbon”
The name “carbohydrate” originated from the observation that many simple sugars fit the formula Cx(H2O)y. This suggested a structure of carbon atoms combined with water molecules. Glucose (C6H12O6) fits this pattern perfectly.
This rule fails upon closer inspection. Acetic acid (CH3COOH) fits the formula but is not a carbohydrate. Rhamnose (C6H12O5) is a carbohydrate but does not fit the formula.
Correct Definition
Optically active polyhydroxy aldehydes or ketones, or compounds which produce such units on hydrolysis.
2. The “Inverting” Trick of Sucrose
Sucrose (table sugar) is dextrorotatory, meaning it rotates polarized light to the right. When hydrolyzed, it splits into glucose and fructose. The resulting mixture behaves differently. Glucose rotates light to the right (+52.5°), but fructose rotates light strongly to the left (–92.4°). The strong leftward rotation of fructose overpowers the glucose. The mixture becomes laevorotatory. This specific mixture is called Invert Sugar.
3. Cooking Only Changes Shape, Not Sequence
When you boil an egg, the white coagulates. This is denaturation. Heat disturbs the hydrogen bonds holding the protein chains in their specific 3D shape (globules and helices). The chains unfold. The biological activity is lost, but the chemical identity remains. The primary structure—the sequence of amino acids—remains intact. The blueprint survives the destruction of the building.
4. “Vitamin” is a Historical Typo
The term began as “Vitamine” (Vital + Amine) because early researchers thought all these compounds contained amino groups. Later research proved this false. The ‘e’ was dropped, leaving us with “Vitamin.” The name is a fossil of early scientific understanding.
Core Concepts & Analysis
Deep dive into structure, function, and reaction mechanisms.
Carbohydrates: Structure & Classification
Classification Logic
Carbohydrates classify based on hydrolysis products. Use the tabs below to view examples.
- Cannot be hydrolyzed further.
- Examples: Glucose, Fructose, Ribose.
- All are reducing sugars.
- Yield 2-10 monosaccharide units.
- Disaccharides: Sucrose (Glc + Fru), Maltose (Glc + Glc), Lactose (Glc + Gal).
- Sucrose is non-reducing; Maltose/Lactose are reducing.
- Yield large number of units.
- Examples: Starch, Cellulose, Glycogen.
- Non-reducing and generally tasteless.
Isomers differing only in configuration at C1 (the hemiacetal carbon). α-Glucose and β-Glucose are classic examples.
Visualizing Glucose
Canvas RenderSchematic: Fisher Projection converting to Pyranose ring
Disaccharides: The Double Sugars
Interactive TreeVisualizing breakdown products (Click nodes to expand/collapse)
| Sugar | Components | Reducing Nature |
|---|---|---|
| Sucrose | α-D-Glucose + β-D-Fructose | Non-Reducing |
| Maltose | α-D-Glucose + α-D-Glucose | Reducing |
| Lactose | β-D-Galactose + β-D-Glucose | Reducing |
Polysaccharides: Nature’s Energy Tanks
Interactive Data1. Starch Composition: Amylose vs Amylopectin
Starch is the main storage polysaccharide in plants.
Hover over segments for details
2. Glycogen (Animal Starch)
The storage polysaccharide in animal body.
3. Cellulose
Most abundant organic substance in plant kingdom.
Advanced Analysis: Synthesis & Structure
Lab Report View1. Preparation of Glucose
From Sucrose (Cane Sugar)
Process: Boiled with dilute HCl or H₂SO₄ in alcoholic solution.
Result: Equimolar mixture obtained.
From Starch
Process: Hydrolysis with dilute H₂SO₄ at 393 K under 2-3 atm pressure.
Scale: Industrial production.
2. Amino Acids: Dietary Classification
| Category | Definition | Examples |
|---|---|---|
| Essential | Cannot be synthesized by the body; must be in diet. | Valine, Leucine, Isoleucine, Lysine |
| Non-Essential | Can be synthesized by the body. | Glycine, Alanine, Glutamic Acid |
Zwitter Ion Concept
In aqueous solution, the carboxyl group loses a proton and the amino group accepts it, creating a dipolar ion.
Proteins: Architecture of Life
| Feature | Fibrous Proteins | Globular Proteins |
|---|---|---|
| Shape | Linear, fiber-like structure. | Spherical, folded structure. |
| Solubility | Insoluble in water. | Soluble in water. |
| Forces | Hydrogen & Disulphide bonds. | Weak intermolecular forces. |
| Examples | Keratin (hair), Myosin (muscle). | Insulin, Albumins. |
Protein Structure: Levels of Complexity
D3 HierarchyMapping forces and features across levels
Secondary Structure: The Two Motifs
| Property | α-Helix | β-Pleated Sheet |
|---|---|---|
| Shape | Right-handed screw (Coil) | Extended, flat sheet |
| H-Bonds | Intramolecular (Within chain) | Intermolecular (Between chains) |
Visualizing α-Helix
Schematic representation
Enzymes: Biological Catalysts
Enzymes differ from standard inorganic catalysts. They are highly specific, work under mild temperature/pH conditions, and regulate metabolic pathways efficiently.
Key Characteristics
- Specificity: One enzyme catalyzes one specific reaction (e.g., Urease only hydrolyzes urea).
- Efficiency: Can increase reaction rates by factors of 1020.
- Optimum Conditions: Function best at specific pH (usually 5-7) and Temperature (37°C).
Mechanism (Lock & Key)
1. Enzyme (E) binds Substrate (S) → [E-S] Complex.
2. Product formation → [E-P] Complex.
3. Release → Enzyme (E) + Product (P).
Vitamins & Hormones: Chemical Regulators
Vitamins: Essential Micronutrients
Stored in liver & adipose tissues. Can accumulate in the body.
Excreted in urine. Must be replenished regularly (except B12).
Hormones: Chemical Messengers
- Steroids: Estrogens, Androgens.
- Polypeptides: Insulin, Glucagon.
- Amino Acid Derivatives: Thyroxine, Adrenaline.
Essential Vitamins Checklist
| Vitamin | Common Sources | Deficiency Diseases |
|---|---|---|
| Vitamin A | Fish liver oil, carrots, butter, milk | Xerophthalmia (hardening of cornea), Night blindness |
| Vitamin B1 (Thiamine) | Yeast, milk, green vegetables, cereals | Beri beri (loss of appetite, retarded growth) |
| Vitamin B2 (Riboflavin) | Milk, egg white, liver, kidney | Cheilosis (fissuring at corners of mouth), digestive disorders |
| Vitamin B6 (Pyridoxine) | Yeast, milk, egg yolk, cereals | Convulsions |
| Vitamin B12 | Meat, fish, egg, curd | Pernicious anaemia (RBC deficiency in haemoglobin) |
| Vitamin C (Ascorbic Acid) | Citrus fruits, amla, green leafy vegetables | Scurvy (bleeding gums) |
| Vitamin D | Exposure to sunlight, fish, egg yolk | Rickets (bone deformities in children), Osteomalacia |
| Vitamin E | Vegetable oils like wheat germ oil, sunflower oil | Increased fragility of RBCs, muscular weakness |
| Vitamin K | Green leafy vegetables | Increased blood clotting time |
Nucleic Acids: The Genetic Blueprint
Interactive SunburstHover center-out to explore Base Classification
Composition Hierarchy
Phosphodiester Linkage
Nucleotides are joined by a linkage between the 5′ carbon of one pentose sugar and the 3′ carbon of the next. This forms the backbone.
Watson-Crick Model (DNA)
- Adenine (A) = Thymine (T) (2 H-bonds)
- Cytosine (C) ≡ Guanine (G) (3 H-bonds)
Interactive Mastery Quiz
Frequently Asked Questions
Why are vitamins essential if we need them in small amounts?
Vitamins act as cofactors for enzymes.
What is the difference between Glycosidic and Peptide linkages?
A Glycosidic linkage joins two monosaccharides via an oxygen atom. A Peptide linkage joins amino acids via an amide group (-CO-NH-).
Glossary

Unit 13 CBSE Chemistry Amines Class 12 Practice Exam with Q&A
Nitrogen-containing organic compounds serve as fundamental building blocks in pharmaceuticals, polymers, and biological systems. This study resource for Class 12 Chemistry Unit 13 examines Amines, categorizing them as alkyl or aryl derivatives of ammonia. The content maps out their pyramidal structure, classification, and physical properties, such as solubility and boiling point variations.
Students will find specific breakdowns of chemical behaviors, including the Carbylamine reaction and Hinsberg’s test, alongside mechanism explanations for Hoffmann Bromamide degradation. We also address the factors influencing basicity in gaseous versus aqueous phases and use interactive visualizers to demonstrate stereochemical concepts like nitrogen inversion.
Rezyo.in
Class XII Unit 13: Amines
Amines: Organic Derivatives of Ammonia
Amines are versatile organic compounds derived by replacing hydrogen atoms of ammonia with alkyl or aryl groups. This guide covers the complete 2025-26 syllabus for Unit 13, including structure, basicity trends, key preparation methods like Gabriel Phthalimide synthesis, and distinguishing tests like Hinsberg’s reagent.
Knowledge Check
Pop-out quiz with timer to test your speed.
Unit 13 Challenge
Test your understanding of basicity orders, name reactions, and chemical tests. You have 25 questions. The clock starts when you click below.
Classification & Structure
Primary
\( R-NH_2 \)
One alkyl/aryl group
Secondary
\( R-NH-R’ \)
Two alkyl/aryl groups
Tertiary
\( R_3N \)
Three alkyl/aryl groups
Key Preparation Methods
Nitrogen Inversion (Umbrella Flip)
Rapid oscillation of the nitrogen atom through the plane of substituents makes isolation of enantiomers impossible at room temperature.
Important Chemical Reactions
Carbylamine Reaction (Isocyanide Test)
Test for 1° AminesHinsberg’s Test (Distinction)
Forms sulphonamide soluble in alkali.
Forms sulphonamide insoluble in alkali.
Does not react with Hinsberg’s reagent.
Frequently Asked Questions
Why are aliphatic amines stronger bases than aromatic amines?
In aromatic amines (e.g., aniline), the lone pair on Nitrogen is involved in resonance with the benzene ring, making it less available for protonation. In aliphatic amines, alkyl groups show +I effect, increasing electron density on N.
Why does aniline not undergo Friedel-Crafts reaction?
Aniline is a Lewis base and reacts with the Lewis acid catalyst (\( AlCl_3 \)) to form a salt. This places a positive charge on N, making it a strong deactivating group.
Why are amines soluble in water?
Lower aliphatic amines can form hydrogen bonds with water molecules. Solubility decreases with increasing molar mass due to the larger hydrophobic alkyl part.
What is the basicity order of amines in aqueous solution?
For ethyl groups: \( 2^\circ > 3^\circ > 1^\circ > NH_3 \).
For methyl groups: \( 2^\circ > 1^\circ > 3^\circ > NH_3 \).
This order depends on inductive effect, solvation effect, and steric hindrance.
Why is aniline acetylated before nitration?
Direct nitration causes oxidation of aniline to tarry products. Acetylation protects the amino group and decreases its activating effect (due to resonance of lone pair with carbonyl), preventing polysubstitution and oxidation.
Why are aromatic diazonium salts more stable than aliphatic ones?
Aromatic diazonium salts are stabilized by resonance dispersal of the positive charge into the benzene ring. Aliphatic diazonium salts lack this stabilization and decompose immediately to liberate nitrogen gas and form alcohols.
CBSE Class 12 Amines Notes: Preparation, Reactions, Nomenclature & Mechanisms
Nitrogenous organic compounds function as essential components in synthesis and biology. This study guide for CBSE Class 12 Chemistry Unit 13 examines Amines, categorizing them as primary, secondary, or tertiary derivatives of ammonia. The content maps out core concepts ranging from orbital hybridization and pyramidal geometry to practical laboratory methods like the reduction of nitro compounds and nitriles.
Try our Practice Test
Students will find specific breakdowns of chemical reactions, including the Carbylamine reaction and Hinsberg’s test, alongside mechanism explanations for Hoffmann Bromamide degradation. The notes also address physical properties, such as boiling point variations, and analyze the resonance stability that differentiates aryl amines from alkyl amines. Sections on spectroscopic identification and nitrogen inversion provide advanced context for understanding molecular behavior.
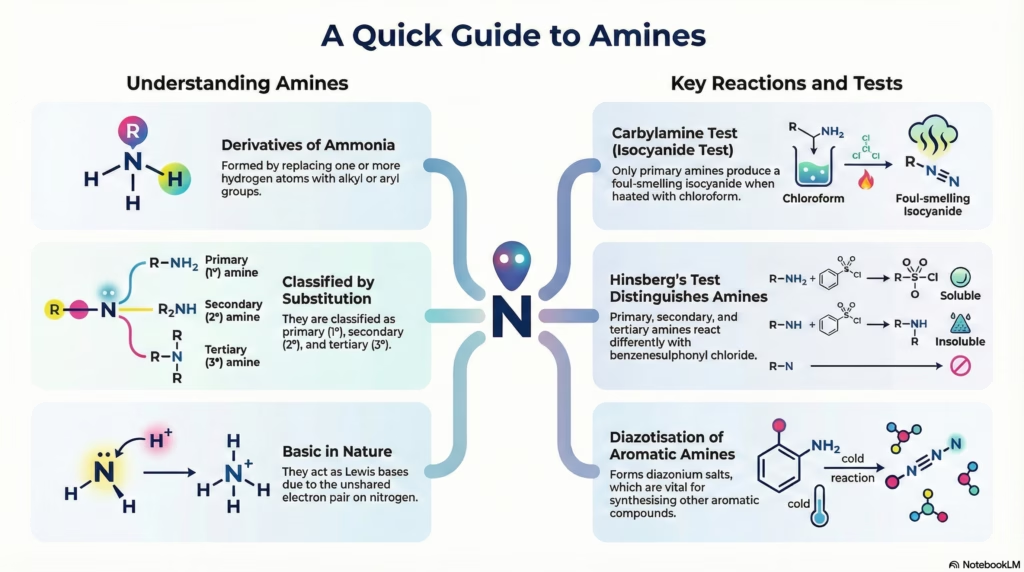
Amines
Comprehensive notes on Structure, Classification, Nomenclature, Preparation, and Properties of Amines for CBSE Class 12.
Contents
1. Introduction
Amines can be considered as derivatives of ammonia (NH3), obtained by replacement of one, two or all the three hydrogen atoms by alkyl and/or aryl groups.
Structure of Amines
Nitrogen has 5 valence electrons. In amines, nitrogen is sp3 hybridised. The shape is pyramidal due to the presence of one lone pair of electrons. The bond angle is slightly less than 109.5° (e.g., 108° in trimethylamine).
2. Classification
One hydrogen atom of ammonia is replaced by an alkyl/aryl group.
Two hydrogen atoms of ammonia are replaced by alkyl/aryl groups.
All three hydrogen atoms of ammonia are replaced by alkyl/aryl groups.
3. Nomenclature
| Structure | Common Name | IUPAC Name |
|---|---|---|
| CH3-NH2 | Methylamine | Methanamine |
| CH3-CH2-NH2 | Ethylamine | Ethanamine |
| CH3-NH-CH3 | Dimethylamine | N-Methylmethanamine |
| C6H5-NH2 | Aniline | Aniline or Benzenamine |
4. Methods of Preparation
1. Reduction of Nitro Compounds
Nitro compounds are reduced to amines by passing hydrogen gas in the presence of finely divided nickel, palladium or platinum and also by reduction with metals in acidic medium.
2. Ammonolysis of Alkyl Halides
An alkyl halide reacts with an ethanolic solution of ammonia to undergo nucleophilic substitution reaction.
R-X + NH3 → R-NH2 → R2NH → R3N → R4N+X–
3. Reduction of Nitriles
Gabriel Phthalimide Synthesis
Important: Only for preparation of primary aliphatic amines. Aromatic primary amines cannot be prepared by this method because aryl halides do not undergo nucleophilic substitution with the anion formed by phthalimide.
Hoffmann Bromamide Degradation
Preparation of primary amines by treating an amide with bromine in an aqueous or ethanolic solution of NaOH. The amine formed contains one carbon less than the parent amide.
5. Physical Properties
Solubility
Lower aliphatic amines are soluble in water because they can form hydrogen bonds with water molecules. Solubility decreases with an increase in molar mass of amines due to an increase in the size of the hydrophobic alkyl part.
Boiling Points
Order of boiling points of isomeric amines:
Primary amines have the highest boiling points due to extensive intermolecular hydrogen bonding.
6. Chemical Reactions
Basic Character of Amines
Amines act as Lewis bases due to the presence of a lone pair of electrons on the nitrogen atom.
Basicity Order in Gaseous Phase:
Tertiary (3°) > Secondary (2°) > Primary (1°) > NH3
Basicity Order in Aqueous Solution (Ethyl group):
2° > 3° > 1° > NH3
(Due to combination of inductive effect, solvation effect, and steric hindrance)
Carbylamine Reaction
Test for Primary Amines only (Aliphatic & Aromatic).
Reaction with Aryl Sulphonyl Chloride (Hinsberg’s Reagent)
Used to distinguish between 1°, 2°, and 3° amines.
- 1° Amine: Forms precipitate soluble in alkali.
- 2° Amine: Forms precipitate insoluble in alkali.
- 3° Amine: Does not react.
7. Electrophilic Substitution
The -NH2 group is ortho and para directing and a powerful activating group.
Bromination
Reacts with bromine water to give 2,4,6-tribromoaniline (White ppt).
Nitration
Direct nitration gives significant amount of meta-derivative along with ortho and para isomers.
Sulphonation
Reacts with H2SO4 to form Zwitter ion (Sulphanilic acid).
8. Mechanisms in Focus
Mechanism: Hoffmann Bromamide Degradation
This reaction involves the migration of an alkyl or aryl group from the carbonyl carbon to the nitrogen atom. The amine formed has one carbon less than the starting amide.
Formation of N-bromoamide
R-CONH2 + Br2 + OH– → R-CONHBr + H2O + Br–
Formation of Acylnitrene Intermediate
Loss of proton from nitrogen by base, followed by loss of bromide ion to form an unstable nitrene species.
Rearrangement (Wolff Rearrangement Analogue)
The alkyl group (R) migrates from Carbon to Nitrogen, forming an Isocyanate (R-N=C=O).
Hydrolysis
R-N=C=O + 2OH– → R-NH2 + CO32-
9. Resonance & Stability
Why is Aniline less basic than Alkylamines?
In aniline, the -NH2 group is attached directly to the benzene ring. The lone pair of electrons on the nitrogen atom enters into conjugation with the benzene ring and is thus less available for protonation.
Resonance Structures of Aniline:
The lone pair delocalizes onto the Ortho and Para positions, creating electron density at these points.
(Neutral)
(Ortho -)
(Para -)
(Ortho -)
(Neutral)
Reactivity Consequence
Since electron density increases at ortho and para positions, electrophiles attack at these locations.
Stability of Anilinium Ion
The anilinium ion formed after accepting a proton has only two Kekule structures and is less stable than the unprotonated aniline (which has 5 resonance structures).
10. Applications & Significance
Synthetic Dyes
Aromatic amines are crucial in the dye industry. Diazonium salts are used to produce Azo dyes (e.g., Methyl Orange, Aniline Yellow) via coupling reactions.
Pharmaceuticals
Amines are biologically active. Sulpha drugs (sulphonamides) derived from benzenesulphonyl chloride are powerful antibacterials. Novocaine is an amine-based anesthetic.
Biological Role
Biologically important amines include Adrenaline and Noradrenaline (neurotransmitters) which regulate blood pressure. Histamine causes allergic reactions.
10. Spectroscopic Identification
Analytical techniques are essential in modern research for identifying amine structures.
Infrared (IR) Spectroscopy
- Region: N-H stretching occurs at 3300–3500 cm-1.
- Primary Amines: Show two bands (symmetric and asymmetric stretching).
- Secondary Amines: Show a single band (weaker).
- Tertiary Amines: Show no absorption in this region (no N-H bond).
NMR Spectroscopy
- Chemical Shift: Protons on the α-carbon are deshielded (2.2–3.0 ppm).
- N-H Protons: Variable chemical shift (0.5–5.0 ppm) due to hydrogen bonding and exchange rate.
- Broadening: Signals often appear broad due to Quadrupole moment of Nitrogen.
11. Nitrogen Inversion
Pyramidal Inversion (Umbrella Flip)
Although nitrogen in amines is sp3 hybridized and chiral when attached to three different groups, individual enantiomers cannot usually be isolated. This is due to rapid pyramidal inversion at room temperature, where the nitrogen atom oscillates through the plane of the substituents.
Note: Quaternary ammonium salts (R4N+) cannot undergo inversion (no lone pair) and thus can be resolved into stable enantiomers if the four groups are different.
12. Study Guide & Quiz
This guide provides a review of key concepts related to amines and diazonium salts. Test your recall with the short-answer quiz below.
Instructions: Click on a question to reveal the answer.
Describe the geometry and orbital hybridization of the nitrogen atom in amines. What is the approximate bond angle and why?
Explain why alkylamines are generally stronger bases than ammonia.
What is the primary limitation of the Gabriel phthalimide synthesis?
How does the Hoffmann bromamide degradation reaction alter the carbon skeleton?
Describe the Carbylamine reaction and its use.
Why is the activating effect of the amino group in aniline often controlled?
Explain why aniline does not undergo Friedel-Crafts reactions.
What are the reaction conditions for the diazotisation of aniline?
What is the key difference between Sandmeyer and Gatterman reactions?
Define a coupling reaction.
13. Essay Questions
1. Basicity Trends
Compare and contrast the basicity of primary, secondary, and tertiary alkylamines in both the gaseous phase and in an aqueous solution. Provide a detailed explanation for the observed trends in each phase, discussing the specific roles of the inductive effect, solvation effect, and steric hindrance.
2. Diazonium Salts Utility
Discuss the synthetic utility of diazonium salts as intermediates in the synthesis of aromatic compounds. Describe at least five distinct types of substitution reactions involving the displacement of the diazonium group, providing the specific reagents required for each transformation.
3. Electrophilic Substitution Challenges
Explain the chemical challenges encountered during the direct electrophilic substitution (specifically nitration and bromination) of aniline. Detail the chemical strategy used to overcome these challenges to produce monosubstituted products, explaining how the protecting group modifies the reactivity of the aromatic ring.
4. Chemical Tests
Describe a series of chemical tests that could be used to definitively distinguish between an unknown primary amine, secondary amine, and tertiary amine. For each test (e.g., Hinsberg’s test, Carbylamine test), explain the reagents used, the expected chemical reaction, and the observable results for each class of amine.
5. Preparation Methods
Elaborate on three different methods for the preparation of primary amines: Reduction of Nitro Compounds, Gabriel Phthalimide Synthesis, and Hoffmann Bromamide Degradation. For each method, detail the starting materials, reagents, and any significant advantages, disadvantages, or unique features (e.g., effect on carbon chain length).
14. Glossary of Key Terms
- Acylation
- A reaction where aliphatic or aromatic primary and secondary amines react with acid chlorides, anhydrides, and esters. It involves the replacement of a hydrogen atom of the -NH2 or >N-H group by an acyl group to form an amide.
- Amine
- An important class of organic compounds derived by replacing one or more hydrogen atoms of an ammonia molecule with alkyl and/or aryl groups.
- Ammonolysis
- The process of cleavage of a C-X (carbon-halogen) bond by an ammonia molecule. It is a nucleophilic substitution reaction where an alkyl or benzyl halide reacts with an ethanolic solution of ammonia to replace the halogen with an amino (-NH2) group.
- Arylamine
- An amine in which the -NH2 group is directly attached to a benzene ring. Aniline (C6H5NH2) is the simplest example.
- Azo Compounds
- Coloured compounds that have an extended conjugate system with two aromatic rings joined through an -N=N- bond. They are used as dyes and are formed via coupling reactions.
- Basic Character of Amines
- The ability of amines to act as Lewis bases due to the unshared pair of electrons on the nitrogen atom. They react with acids to form salts.
- Benzoylation
- A specific type of acylation reaction where an amine reacts with benzoyl chloride (C6H5COCl).
- Carbylamine Reaction
- Also known as the isocyanide test. A reaction where aliphatic and aromatic primary amines are heated with chloroform and ethanolic potassium hydroxide to form foul-smelling substances called isocyanides or carbylamines. It is used as a test for primary amines.
- Coupling Reaction
- A reaction involving the retention of the diazo group, where a diazonium salt reacts with an electron-rich aromatic compound (like phenol or aniline) to form a coloured azo compound. It is an electrophilic substitution reaction.
- Diazonium Salts
- A class of compounds with the general formula R-N2+X–, where R is an aryl group. The N2+ group is called the diazonium group. They are important intermediates in the synthesis of aromatic compounds.
- Diazotisation
- The process of converting a primary aromatic amine into a diazonium salt by reacting it with nitrous acid at low temperatures (273-278 K).
- Gabriel Phthalimide Synthesis
- A method used for the preparation of primary amines. It involves treating phthalimide with ethanolic KOH, followed by heating with an alkyl halide and subsequent alkaline hydrolysis.
- Gatterman Reaction
- A reaction to introduce chlorine or bromine into a benzene ring by treating a diazonium salt solution with the corresponding halogen acid in the presence of copper powder.
- Hinsberg’s Reagent
- The common name for benzenesulphonyl chloride (C6H5SO2Cl). It is used to distinguish between primary, secondary, and tertiary amines.
- Hoffmann Bromamide Degradation
- A method for preparing primary amines by treating an amide with bromine in an aqueous or ethanolic solution of sodium hydroxide. The resulting amine contains one carbon atom less than the parent amide.
- pKb
- A measure of the basicity of a substance; defined as the negative logarithm of the base dissociation constant (Kb). A smaller pKb value indicates a stronger base.
- Primary (1°) Amine
- An amine formed when one hydrogen atom of ammonia is replaced by an alkyl (R) or aryl (Ar) group, resulting in a structure of the type RNH2 or ArNH2.
- Sandmeyer Reaction
- A reaction where a diazonium group is replaced by Cl–, Br–, or CN– by treating the diazonium salt with the corresponding copper(I) salt.
- Secondary (2°) Amine
- An amine formed when two hydrogen atoms of ammonia are replaced by alkyl or aryl groups, resulting in a structure of the type R-NHR’.
- Sulphonamide
- The product formed when primary or secondary amines react with benzenesulphonyl chloride (Hinsberg’s reagent).
- Tertiary (3°) Amine
- An amine formed when all three hydrogen atoms of ammonia are replaced by alkyl or aryl groups, resulting in a structure of the type R3N.

Class 12 Chemistry Unit 6: Haloalkanes and Haloarenes – Practice Questions (2025-26)
Unit 6 moves beyond basic hydrocarbons to explore functional group reactivity. By replacing hydrogen atoms with halogens, we generate compounds with distinct properties driven by the polarized Carbon-Halogen bond.
This guide examines the logic behind Nucleophilic Substitution, clarifying the differences between SN1 and SN2 pathways, while also breaking down the stereochemical principles of chiral molecules. It covers essential preparation methods, chemical properties, and the environmental effects of polyhalogen compounds like DDT and Freons.
Rezyo.in
Class XII Unit 6: Haloalkanes & Haloarenes
Haloalkanes & Haloarenes: Reactivity & Structure
Unit 6 bridges hydrocarbon chemistry with functional group reactivity. This guide explores how the polarized Carbon-Halogen bond drives reactions like Nucleophilic Substitution ($S_N1/S_N2$) and Elimination, while also addressing the environmental impact of polyhalogen compounds.
Concept Check
Pop-out quiz to test your understanding of mechanisms and reagents.
Organic Chemistry Challenge
Ready to test your mastery? Identify reagents, predict products, and distinguish between SN1 and SN2 pathways.
Nature of C-X Bond
Halogens are more electronegative than carbon, creating a permanent dipole. The carbon acquires a partial positive charge ($\delta+$), making it an electrophilic center susceptible to nucleophilic attack.
- Bond Polarity: C-F > C-Cl > C-Br > C-I
- Bond Length: C-F < C-Cl < C-Br < C-I
- Reactivity: R-I > R-Br > R-Cl > R-F (Weakest bond breaks first)
Bond Enthalpy (kJ/mol)
Methods of Preparation
From Alcohols
Best Method-
Thionyl Chloride:
R-OH + SOCl2 → R-Cl + SO2↑ + HCl↑Byproducts are gases (pure product).
-
Lucas Reagent:
R-OH + HCl (ZnCl2) → R-ClOrder: 3° > 2° > 1°
From Hydrocarbons
-
Free Radical Halogenation:
Alkane + Cl2 (UV) → MixturePoor yield of single isomer.
-
Electrophilic Addition:
Alkene + HX → Alkyl HalideFollows Markovnikov’s Rule.
Halogen Exchange
-
Finkelstein (Iodides):
R-X + NaI → R-I + NaXSolvent: Dry Acetone (ppt forms).
-
Swarts (Fluorides):
R-Br + AgF → R-F + AgBrReagents: AgF, Hg2F2, SbF3.
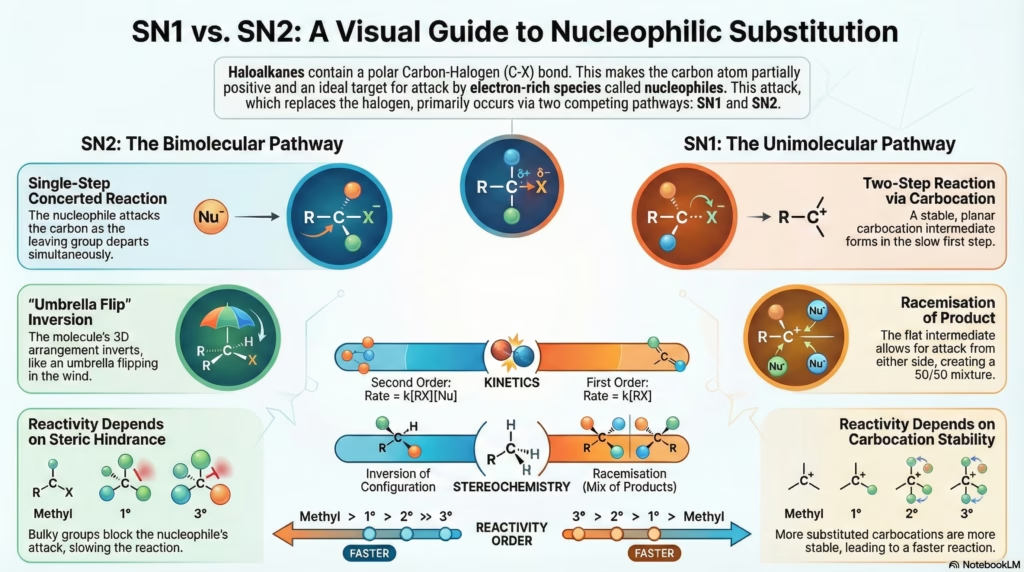
Reaction Mechanisms: SN1 vs SN2
SN2 (Bimolecular)
- Kinetics: Rate = k[Substrate][Nu]
- Step: Single concerted step.
- Stereochem: Inversion (Walden).
- Order: Methyl > 1° > 2° > 3°
SN1 (Unimolecular)
- Kinetics: Rate = k[Substrate]
- Step: Two steps (Carbocation).
- Stereochem: Racemization.
- Order: 3° > 2° > 1° > Methyl
Stereochemistry Essentials
Chirality
Objects non-superimposable on their mirror image. Usually implies an asymmetric carbon bonded to 4 different groups.
Enantiomers
Stereoisomers that are mirror images. They have identical physical properties but rotate light in opposite directions.
Racemic Mixture
A 50:50 mixture of two enantiomers. Optically inactive due to external compensation.
Left Hand
Right Hand (Mirror)
Reactivity of Haloarenes
Why Low Reactivity?
Aryl halides are extremely less reactive towards Nucleophilic Substitution due to:
- Resonance Effect: Partial double bond character in C-X bond makes cleavage difficult.
- Hybridization: sp2 carbon holds the halogen more tightly than sp3.
- Instability: Phenyl cation is unstable (rules out SN1).
Dow’s Process (Forcing conditions)
Chlorobenzene can be converted to Phenol using NaOH at 623 K and 300 atm.
Effect of -NO2
Electron withdrawing groups at ortho/para positions increase reactivity by stabilizing the intermediate carbanion.
Polyhalogen Compounds
DDT
First chlorinated organic insecticide. Banned due to bio-magnification.
Freons (CFCs)
Refrigerants. Cause ozone layer depletion in stratosphere.
Chloroform
Stored in dark bottles to prevent formation of poisonous Phosgene (COCl2).
Iodoform
Earlier used as antiseptic but replaced due to smell. Activity due to free Iodine.
CCl4
Carbon Tetrachloride. Used as feedstock for refrigerants. Exposure causes liver cancer.
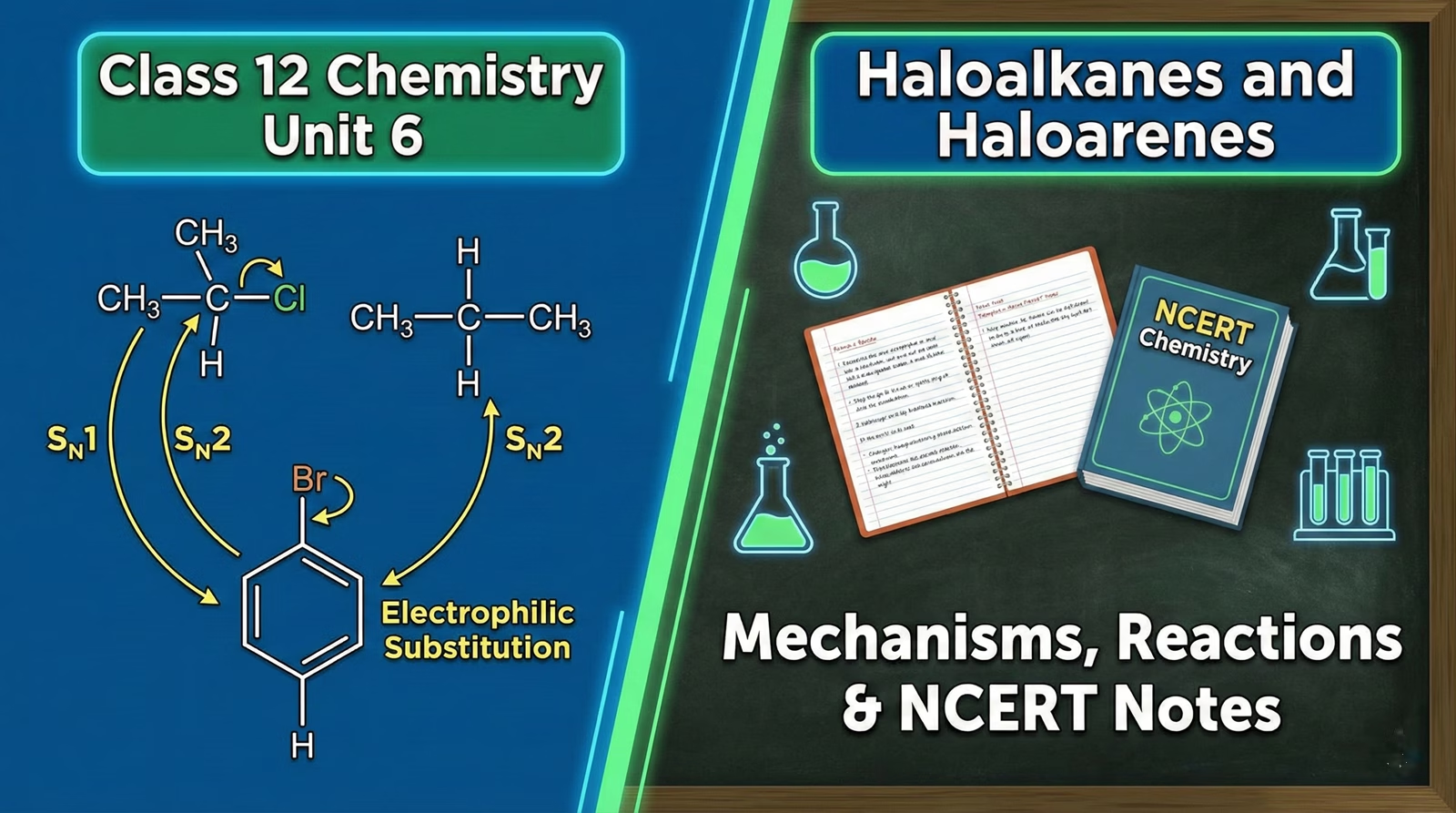
Class 12 Chemistry Unit 6: Haloalkanes and Haloarenes – Mechanisms, Reactions & NCERT Notes
Unit 6: Haloalkanes and Haloarenes often serves as the entry point into the complex world of Class 12 Organic Chemistry. Many students struggle to connect the theoretical nature of the C-X bond with practical application in reaction mechanisms like SN1 and SN2. Instead of rote memorization, this study module focuses on the logic behind the reactions.
We break down the syllabus into manageable sections, covering everything from the specific conditions required for Sandmeyer’s reaction to the stereochemical implications of nucleophilic substitution. You will find visual comparisons for boiling point trends, a clear roadmap for solving organic conversions, and a dedicated breakdown of why haloarenes resist substitution. Use the interactive charts and reagent cheat sheets below to solidify your understanding and prepare for board exams.
Haloalkanes and Haloarenes
A complete study guide for Class XII (2025-26 Syllabus). Master organic reaction mechanisms, understand stereochemical principles, and apply logic to solve conversion problems.
1. Nature of C-X Bond
Halogen atoms are more electronegative than carbon. This creates a permanent dipole where carbon is slightly positive (electrophilic) and the halogen is slightly negative.
- Bond Polarity: C-F > C-Cl > C-Br > C-I
- Bond Length: C-F < C-Cl < C-Br < C-I
- Bond Enthalpy: Strongest in C-F, weakest in C-I.
Bond Parameters Visualization
Relative Bond Dissociation Enthalpy (Height)
2. Methods of Preparation
Synthesis Pathways to Haloalkanes (R-X)
From Alcohols (R-OH)
From Hydrocarbons
Halogen Exchange
3. Named Reaction Gallery
Sandmeyer’s Reaction
Preparation of Haloarenes from Diazonium Salts
Uses cuprous halide (Cu2X2) dissolved in corresponding halogen acid.
Gattermann Reaction
Modification of Sandmeyer
Uses Copper powder (Cu) instead of Cuprous halide.
Wurtz-Fittig Reaction
Coupling Alkyl + Aryl
Reaction in presence of dry ether to form Alkylbenzene.
4. Physical Properties Logic
BP Trend Boiling Points
Boiling points depend on van der Waals forces, which depend on molecular mass and surface area.
Visualizing Surface Area
Spherical molecules (highly branched) have less contact area with neighbors, leading to weaker attraction and lower boiling points.
5. SN1 vs SN2 Mechanisms
| Feature | SN2 (Bimolecular) | SN1 (Unimolecular) |
|---|---|---|
| Kinetics | Second order. Rate = k[RX][Nu] | First order. Rate = k[RX] |
| Steps | Single Step (Concerted). | Two Steps (Ionization + Attack). |
| Intermediate | Transition State (Pentacoordinate). | Carbocation (Planar). |
| Stereochemistry | Inversion (Walden Inversion). “Umbrella” flips inside out. | Racemization. Attack from both sides of planar carbocation. |
| Order of Reactivity | Methyl > 1° > 2° > 3° (Steric hindrance controls rate) |
3° > 2° > 1° > Methyl (Carbocation stability controls rate) |
| Solvent | Polar Aprotic (Acetone, DMSO). | Polar Protic (Water, Alcohol). |
6. Reaction Energy Profiles
SN2 Profile (Single Step)
One smooth curve. Reactants go directly to Transition State (TS) and then to Products. No intermediate.
SN1 Profile (Two Steps)
Two distinct humps. The “valley” between them represents the stable Carbocation Intermediate.
7. Stereochemistry Essentials
Key Definitions
-
1
Chirality
Objects (or molecules) that are non-superimposable on their mirror image. Usually contains an asymmetric carbon (bonded to 4 different groups).
-
2
Enantiomers
Stereoisomers that are non-superimposable mirror images. They have identical physical properties but rotate plane-polarized light in opposite directions.
-
3
Racemic Mixture
A 1:1 mixture of two enantiomers. It has zero optical rotation because the rotation of one isomer cancels the other.
Chirality Concept: Hands are chiral.
Molecules with 1 chiral center behave just like hands.
8. Elimination Reactions (Dehydrohalogenation)
Reagent Trigger
Alcoholic KOH acts as a strong base, favoring elimination over substitution.
(Note: Aqueous KOH favors substitution to form Alcohol)
Zaitsev (Saytzeff) Rule
“In dehydrohalogenation reactions, the preferred product is that alkene which has the greater number of alkyl groups attached to the doubly bonded carbon atoms.”
Minor Product: Less substituted alkene
9. Master Reaction Map
The central hub of Alkyl Halide (R-X) Chemistry
10. Reagent Cheat Sheet
Quick reference for common reagents and ambident nucleophiles.
KOH (Potassium Hydroxide)
Cyanide Group (CN)
Nitrite Group (NO₂)
Reaction with Metals
11. Haloarenes Reactivity
Why are Haloarenes unreactive towards Nucleophilic Substitution?
Lone pairs on halogen delocalize into the benzene ring, creating partial double bond character in the C-X bond.
Carbon is sp2 hybridized (more electronegative) compared to sp3 in alkyl halides, holding the halogen tighter.
Deep Dive Forcing the Unreactive: Dow’s Process & Effect of NO₂
While typically unreactive, nucleophilic substitution can occur under drastic conditions or with electron-withdrawing groups.
623 K, 300 atm → Phenol
High temperature and pressure are required to break the strong C-Cl bond.
Presence of NO₂ at Ortho/Para positions increases reactivity.
Warm Water → Picric Acid
NO₂ stabilizes the carbanion intermediate.
Electrophilic Substitution Logic
Why are Halogens Ortho-Para directing despite being deactivating?
+M Effect (Resonance): The lone pair on Halogen donates electrons to the ring, increasing electron density specifically at Ortho and Para positions.
-I Effect (Inductive): The electronegative halogen pulls electrons, deactivating the overall ring.
Result: Reactivity is lower than benzene, but substitution happens at O/P positions.
12. Electrophilic Substitution Gallery
Chlorine is Ortho-Para directing but deactivating. Para product is usually Major due to symmetry.
Halogenation
Cl₂ / Anhyd. FeCl₃Nitration
HNO₃ / H₂SO₃Sulphonation
Conc. H₂SO₄ / HeatFriedel-Crafts
CH₃Cl / Anhyd. AlCl₃13. Conversion Roadmap
Use these standard pathways to solve conversion problems.
Ascent Increasing Carbon Chain Length
Transform Common Transformations
14. Strategic Problem Solving
How to solve “Identify A, B, C”?
Look for the End Product: If the end product is an acid, the precursor was likely a Nitrile or Alcohol.
Count the Carbons: If Carbon count increases, look for KCN or Grignard. If it stays same, standard substitution.
Check for Isomers: If the question mentions “optical activity” or “racemic mixture”, think SN1 mechanism.
Example Logic Flow
15. Common Exam Pitfalls
⚠️ Dont’s
- Don’t forget to account for Hydride/Methyl shifts in SN1 carbocations.
- Don’t use aqueous KOH if you want an Alkene (Use Alcoholic!).
- Don’t assume Chlorobenzene undergoes substitution easily.
✅ Do’s
- Do write “Major” and “Minor” products in Electrophilic substitution.
- Do mention “Inversion of configuration” for SN2 questions.
- Do check if the halide is vinylic or aryl (Low Reactivity).
16. Polyhalogen Compounds
Chloroform (CHCl3)
Used as a solvent. Slowly oxidizes in air/light to form poisonous Phosgene gas.
Iodoform (CHI3)
Previously used as an antiseptic. The antiseptic action is due to the liberation of free Iodine, not the compound itself.
Freons (CFCs)
Stable, unreactive, non-toxic gases used in aerosol propellants and cooling. Major cause of Ozone Layer Depletion.
17. Conceptual Deep Dive
AgCN is predominantly covalent. The silver atom blocks the carbon atom, leaving only the Nitrogen lone pair available for nucleophilic attack, resulting in an Isocyanide (R-NC).
18. Interactive Q&A Practice
Why are haloalkanes insoluble in water despite the C-X bond being polar?
Why is sulphuric acid (H2SO4) not used during the reaction of alcohols with KI?
Which compound reacts faster in SN1 reaction: 2-Bromo-2-methylpropane or 2-bromopropane? Why?
Explain why Grignard reagents should be prepared under anhydrous conditions.
Identify the product formed when Chlorobenzene is treated with Sodium in the presence of dry ether.
2C6H5Cl + 2Na → C6H5-C6H5 + 2NaCl
Environmental Awareness
While Polyhalogen compounds like Freons were industrial milestones, their stability led to Ozone layer depletion. Modern chemistry prioritizes environmental safety alongside utility.
Class 12 Coordination Compounds (Unit 5): Notes, CFT Visualizer & Practice Questions
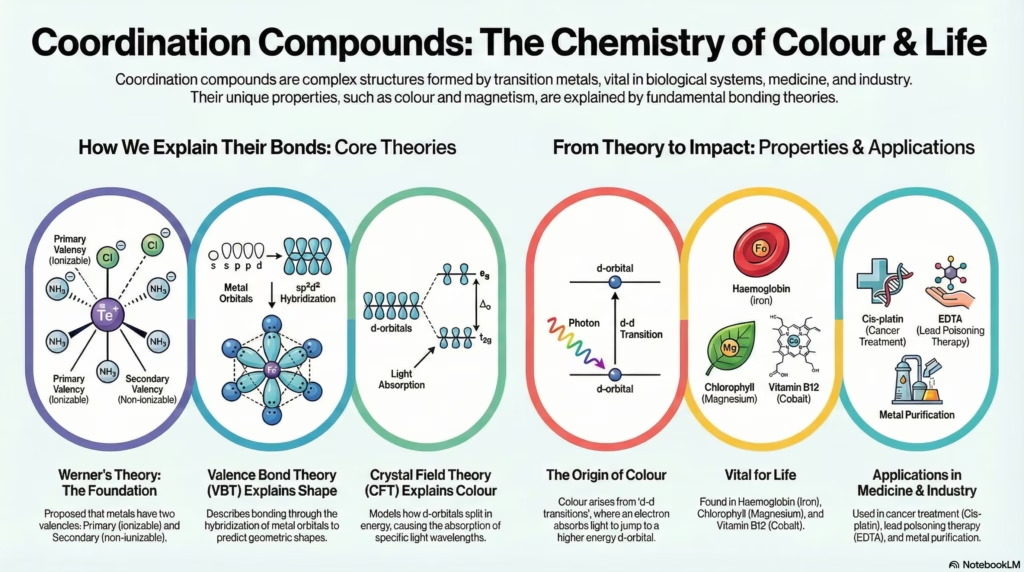
Transition metals possess a unique ability to bind with neutral molecules or anions to form complex structures known as coordination compounds. Unlike simple double salts that break down completely in water, these entities retain their identity and distinct physical properties.
This guide breaks down the core principles governing these structures, starting from Alfred Werner’s early postulates to modern bonding theories. Students will examine how Crystal Field Theory explains the vivid colours seen in transition metal complexes and how Valence Bond Theory predicts their geometry and magnetic behavior.
The included interactive modules and timed assessments provide a practical way to master IUPAC nomenclature and stereoisomerism for the upcoming board exams.
Rezyo.in
Class XII Unit 5: Coordination Compounds
Coordination Compounds: The Chemistry of Colour
Beyond simple salts lies the complex world of Coordination Chemistry. This unit bridges ionic bonding and molecular structure, explaining how transition metals bind with ligands to form colourful, magnetic, and biologically vital compounds like Haemoglobin and Cisplatin.
Knowledge Check
Pop-out quiz with timer to test your grasp on VBT, CFT, and Nomenclature.
Coordination Mastery Challenge
Ready to test your understanding of Ligands, Isomerism, and Bonding Theories? You have 35 questions. The clock starts when you click below.
Fundamental Terminology
Coordination Entity
The central metal atom bonded to a fixed number of ions or molecules.
Ligands
Lewis bases donating electron pairs to the metal. Classified by denticity.
Concept: Ambidentate Ligands
Ligands with two potential donor atoms but which bond through only one at a time.
Examples:
NO2–: Bond via N (Nitro) or O (Nitrito).
SCN–: Bond via S (Thiocyanato) or N (Isothiocyanato).
IUPAC Nomenclature Rules
- Order: Cation named first, then Anion.
- Alphabetical: Ligands are named alphabetically before the metal.
- Prefixes: Use di-, tri- for simple ligands; bis-, tris- for complex ligands (like ‘en’).
- Metal Ending: If complex is anionic, metal ends in -ate (e.g., Ferrate).
| Formula | Analysis | IUPAC Name |
|---|---|---|
| [Co(NH3)6]Cl3 | Cationic complex. Co(III). | Hexaamminecobalt(III) chloride |
| K3[Fe(C2O4)3] | Anionic complex. Fe(III). | Potassium trioxalatoferrate(III) |
| [Ni(CO)4] | Neutral. Ni(0). | Tetracarbonylnickel(0) |
Valence Bond Theory (VBT)
Predicts geometry based on hybridization of metal orbitals.
CFT: Crystal Field Splitting
Splitting of d-orbitals in Octahedral Field
Isomerism
Structural Isomerism
- Linkage: Ambidentate ligands (NO2 vs ONO).
- Solvate: Water inside/outside sphere ([Cr(H2O)6]Cl3 vs [Cr(H2O)5Cl]Cl2.H2O).
- Ionization: Exchange of counter ions (SO4 vs Br).
- Coordination: Exchange between cationic/anionic complexes.
Stereoisomerism
- Geometrical: Cis (Adjacent) vs Trans (Opposite). Common in square planar and octahedral.
- Optical: Enantiomers (Non-superimposable mirror images). Common in octahedral complexes with chelating ligands (e.g., [Co(en)3]3+).
Formula Cheat Sheet
n = unpaired electrons
Plus Pairing Energy (P) if needed
Tetrahedral vs Octahedral
Conceptual Doubts (FAQ)
Q: Why are Zn2+ complexes colourless?
Zn2+ has a 3d10 configuration (completely filled d-orbitals). There are no empty orbitals for electrons to transition into (no d-d transition possible), hence no visible light is absorbed.
Q: What is the Chelate Effect?
Complexes containing chelating (ring-forming) ligands like ‘en’ or EDTA are significantly more stable than those with monodentate ligands. This is an entropy-driven process.
Q: How does the Spectrochemical Series work?
It arranges ligands by field strength. Weak ligands (Halogens) cause small splitting (High Spin). Strong ligands (CN, CO) cause large splitting (Low Spin/Pairing).
I– < F– < H2O < NH3 < CN– < CO
Q: Why is CO a stronger ligand than NH3?
CO engages in Synergic Bonding. It acts as a sigma donor (C to Metal) and a pi acceptor (Metal to C), strengthening the bond via back-donation.
Chapter Summary
We have explored the architecture of coordination compounds. From Werner’s primary valencies to the orbital splitting of CFT, you now have the tools to predict geometry, magnetic behavior, and colour. Remember: Geometry depends on Hybridization (VBT), but Colour depends on Splitting (CFT).
Coordination Compounds Class 12 Notes Unit 5: Nomenclature, Isomerism, VBT & CFT Explained
Transition metals possess the unique ability to form complex structures known as coordination compounds. Unlike simple double salts such as Carnallite which dissociate completely in water, coordination entities retain their identity due to strong metal-ligand coordinate bonds.
This unit covers the fundamental principles governing these structures, starting with Werner’s postulates and moving through IUPAC nomenclature rules.
Students will examine the geometry and magnetic properties of complexes using Valence Bond Theory (VBT) and understand colour mechanisms via Crystal Field Theory (CFT).
Coordination Compounds
Transition metals form structures known as coordination compounds. Unlike double salts which dissociate completely in water (like Carnallite), coordination compounds retain their identity. The central metal ion binds to ligands via coordinate bonds.
Werner’s Theory
Alfred Werner proposed that metals in coordination compounds exhibit two types of valencies. This theory laid the foundation for modern coordination chemistry.
1. Primary Valency
- Corresponds to the Oxidation State.
- Ionizable (satisfied by anions).
- Represented by dotted lines.
- Example: In CoCl3·6NH3, 3 Cl– ions satisfy primary valency.
2. Secondary Valency
- Corresponds to the Coordination Number.
- Non-ionizable (satisfied by neutral molecules or anions).
- Represented by solid lines.
- Example: In CoCl3·6NH3, 6 NH3 satisfy secondary valency.
[Co(NH3)6]Cl3 + 3AgNO3 → 3AgCl (ppt)
Basics & Terminology
The Coordination Sphere
Click “Re-Draw” to visualize the structure of [Co(NH3)6]Cl3
Coordination Entity
The central metal atom or ion bonded to a fixed number of ions or molecules. In [CoCl3(NH3)3], the entity is the Cobalt ion surrounded by three chloride ions and three ammonia molecules.
Ligands
Ions or molecules bound to the central atom. They act as Lewis bases donating electron pairs.
| Type | Description | Examples |
|---|---|---|
| Monodentate | One donor atom | H2O, NH3, Cl– |
| Bidentate | Two donor atoms | en (H2NCH2CH2NH2), C2O42- |
| Polydentate | Multiple donor atoms | EDTA4- (Hexadentate) |
IUPAC Nomenclature
Cation is named first. Anion second. Within the complex entity, ligands are named alphabetically before the metal.
Anionic ligands end in -o (e.g., Chlorido). Neutral ligands keep names (exceptions: Aqua, Ammine, Carbonyl).
If the complex is anionic, the metal name ends in -ate (Ferrate, Argentate). Oxidation state follows in parentheses (II).
Common Examples
| Formula | Thinking Process | IUPAC Name |
|---|---|---|
| [Co(NH3)6]Cl3 | Cationic complex. Ligand: Ammine (x6). Metal: Cobalt. Ox. State: +3. | Hexaamminecobalt(III) chloride |
| K3[Fe(C2O4)3] | Anionic complex. Counter ion: Potassium. Ligand: Oxalato. Metal: Ferrate. | Potassium trioxalatoferrate(III) |
| [Pt(NH3)2Cl(NO2)] | Neutral. Alphabetic: Ammine, Chlorido, Nitrito-N. | Diamminechloridonitrito-N-platinum(II) |
Isomerism
Structural Isomerism
Linkage Isomerism
Occurs with ambidentate ligands.
Example: [Co(NH3)5(NO2)]Cl2 (Yellow) vs [Co(NH3)5(ONO)]Cl2 (Red)
Solvate Isomerism
Water molecules exchange between coordination sphere and outside lattice.
Example: [Cr(H2O)6]Cl3 (Violet) vs [Cr(H2O)5Cl]Cl2.H2O (Grey-Green)
Ionization Isomerism
Exchange of counter ion and ligand.
Example: [Co(NH3)5SO4]Br vs [Co(NH3)5Br]SO4
Stereoisomerism
Geometrical: Cis vs Trans
Geometric: Different spatial arrangement of ligands. Common in square planar and octahedral complexes.
Optical: Non-superimposable mirror images (Enantiomers). Occurs in octahedral complexes with chelating ligands like [Co(en)3]3+.
Theories of Bonding
Valence Bond Theory (VBT)
- Focuses on orbital hybridization (sp3, dsp2, etc.).
- Explains geometry and magnetic properties.
- Distinguishes Inner Orbital (d2sp3) vs Outer Orbital (sp3d2) complexes.
- Does not explain colour or spectra well.
Crystal Field Theory (CFT)
- Electrostatic model. Ligands are point charges.
- Explains colour via d-d transitions.
- Describes splitting of d-orbitals into t2g and eg sets.
- Introduces Crystal Field Stabilization Energy (CFSE).
Crystal Field Splitting (Octahedral)
Watch how d-orbitals split in the presence of ligands. Electrons jump from lower (t2g) to higher (eg) energy levels by absorbing light.
Octahedral vs Tetrahedral Splitting
Ligands approach along the axes. Orbitals on axes (dx2-y2, dz2) are repelled more.
Result: eg (high) & t2g (low)
Ligands approach between axes. Splitting is inverted and smaller.
Result: t2 (high) & e (low)
Δt = (4/9) Δo
Colour in Coordination Compounds
Complementary Colour Wheel
The observed colour is the complement of the absorbed colour.
Why do they exhibit colour?
It arises from d-d transitions. When white light falls on the complex, electrons in lower d-orbitals absorb specific wavelengths to jump to higher d-orbitals. The remaining light is transmitted.
Example: [Ti(H2O)6]3+
- Absorbs: Yellow-Green light.
- Transmits: Violet (its complement).
- If ligands are removed (e.g., heating), the splitting disappears, and the substance becomes colourless.
Carbonyls & Magnetism
Metal Carbonyls & Synergic Bonding
Organometallic compounds where Carbon Monoxide (CO) acts as a ligand are called Metal Carbonyls (e.g., [Ni(CO)4]). The stability of these complexes arises from the Synergic Effect.
- 1 Sigma (σ) Bond: Lone pair from Carbon donates to the empty d-orbital of the Metal.
- 2 Pi (π) Back-Bond: Filled d-orbital of Metal donates electrons back into the empty anti-bonding π* orbital of Carbon.
Magnetic Properties
The magnetic moment depends on the number of unpaired electrons (n).
Formula: μ = √n(n+2) BM (Bohr Magneton)
Diamagnetic: All electrons are paired.
Spin Only Magnetic Moment Calculator
Applications
Medicine
- Cis-platin: [PtCl2(NH3)2] effectively inhibits the growth of tumors (Cancer treatment).
- EDTA: Used in treating lead poisoning.
Biological Systems
- Haemoglobin: Red pigment of blood, a complex of Iron (Fe).
- Chlorophyll: Green pigment in plants, a complex of Magnesium (Mg).
- Vitamin B12: Cyanocobalamin, a complex of Cobalt (Co).
Extraction & Analysis
- Gold/Silver Extraction: Uses cyanide complexes [M(CN)2]–.
- Water Hardness: Estimated using EDTA titration (Ca2+ and Mg2+ complexes).
- Mond Process: Nickel purification via [Ni(CO)4].
Stability of Coordination Compounds
The stability in solution refers to the equilibrium between the complex and its constituent ions.
Stability Constant (β): Higher values indicate greater stability.
M + 4L ⇌ ML4
Chelate Effect: Complexes formed by chelating ligands (ring-forming like ‘en’) are more stable than those formed by monodentate ligands.
Q&A and FAQ
Practice Problems
1. Why is [Ti(H2O)6]3+ violet?
2. Double Salt vs Complex?
3. Geometry of [Ni(CN)4]2-?
4. Oxidation state of Co in [Co(en)3]3+?
Frequently Asked Questions
Why are Zn2+ complexes colourless?
How do I distinguish between Inner and Outer orbital complexes?
- Inner Orbital: Uses (n-1)d orbitals (e.g., d2sp3). Usually formed with strong field ligands (Low spin).
- Outer Orbital: Uses nd orbitals (e.g., sp3d2). Usually formed with weak field ligands (High spin).
What is the Spectrochemical Series?
I– < Br– < Cl– < F– < OH– < H2O < NH3 < en < CN– < CO CO causes maximum splitting (strongest), while I– causes minimum (weakest).
Chapter Summary
Unit 5 Complete • Class XII Chemistry
Class XII Chemistry Unit 4: d & f Block Elements | Practice Q&A & Trends (2025-26)
Transition and Inner Transition elements define the middle section of the periodic table, presenting a unique set of properties driven by filled and half-filled subshells. This guide breaks down Unit 4 for the 2025-26 curriculum, focusing on the electronic anomalies of the 3d series and the chemical consequences of the Lanthanoid Contraction.
We map out the trends in ionization enthalpy, visualize the geometry of chromate ions, and detail the industrial preparation of key oxidizers. Engage with the interactive modules to calculate magnetic moments and solve high-yield reasoning questions designed for board exam readiness.
Rezyo.in
Class XII Chemistry Unit 4 • 2025-26
d- and f-Block Elements Masterclass
Master the nuances of Transition and Inner Transition elements. From electronic anomalies to the Lanthanoid contraction, test your knowledge against the 2025-26 board curriculum.
Ready to Test Your Knowledge?
Enter the MCP (Master Control Panel) to attempt 25 high-yield Board Exam questions. Features live timer, instant feedback, and detailed explanations.
Spin-Only Magnetic Moment Visualizer
The magnetic character of transition metals is determined by the number of unpaired electrons (n). The formula is:
The Lanthanoid Contraction Effect
Why are Zr and Hf so hard to separate?
Normally, atomic size increases down a group. However, the 14 lanthanoid elements (filling the 4f subshell) intervene before the 5d series. The 4f electrons shield the nucleus very poorly. This results in a higher effective nuclear charge (Zeff) pulling the outer electrons in.
Despite having an extra shell, Hf is virtually the same size as Zr, leading to near-identical chemical properties.
Conceptual Deep Dive
Q: Why are Zn, Cd, and Hg not considered “Transition Elements”?
By definition, a transition element must have an incompletely filled d-subshell in its ground state or stable oxidation state. Group 12 elements (Zn, Cd, Hg) have a full d10 configuration (3d10 4s2 for Zn) in both their ground state and their common +2 ions. Thus, they lack the characteristic properties (colored ions, paramagnetism) of true transition metals.
Q: Why is Cr2+ reducing while Mn3+ is oxidizing? Both are d4.
Cr2+ acts as a reducing agent to become Cr3+ (d3), which is highly stable in aqueous solution due to the half-filled t2g level (t2g3). Conversely, Mn3+ is a strong oxidizing agent because gaining an electron converts it to Mn2+ (d5), which has a stable half-filled d-subshell.
Q: Why do transition metals exhibit variable oxidation states?
This is due to the very small energy gap between the (n-1)d and ns orbitals. Electrons from both subshells can participate in bonding. Unlike non-transition elements (where states differ by 2), transition metal oxidation states differ by 1 unit (e.g., Fe2+ and Fe3+).
Q: What happens when pH changes for Dichromate ions?
Chromates (CrO42-, yellow) and Dichromates (Cr2O72-, orange) exist in equilibrium dependent on pH. In acidic medium (low pH), the equilibrium shifts to form Dichromate (Orange). In alkaline medium (high pH), it shifts to form Chromate (Yellow).
2CrO₄²⁻ (Yellow) + 2H⁺ ⇌ Cr₂O₇²⁻ (Orange) + H₂O
Chapter Summary & Outcomes
You have navigated the architectural framework of the d- and f-block elements. From understanding the stability of half-filled subshells to the industrial applications of Potassium Permanganate, these concepts are pivotal for the board exams.
