Class XII Chemistry Unit 4: d and f Block Elements | Trends, Compounds & Exam Q&A (2025-26)
Transition and Inner Transition elements often present a challenge due to their variable oxidation states and irregular atomic trends. This guide targets the core concepts of Unit 4 for the 2025-26 Class XII curriculum, breaking down the 3d series, Lanthanoids, and Actinoids.
We examine the specific anomalies in melting points and ionization enthalpies, visualize the geometry of chromate and permanganate ions, and outline the reaction pathways for major industrial compounds. Use the interactive modules to track electronic configurations and predict magnetic properties for board exam preparation.
Unit 4: d- and f-Block Elements
A definitive guide to Transition and Inner Transition elements. Updated for the 2025-26 academic curriculum. Explore electronic configurations, magnetic properties, and the nuanced chemistry of Lanthanoids.
The 3d Transition Series
Select an element to inspect its electronic configuration.
Definition Check
A transition element has an incompletely filled d-subshell in its ground state or any common oxidation state.
Zn, Cd, Hg are typically excluded from “true” transition metals because they have full d10 configurations.
Atomic Radii Trends (3d Series)
Notice the initial contraction due to increased nuclear charge, followed by a plateau where electron repulsion counteracts the pull, and finally a slight expansion at Zinc.
Ionization Enthalpy
General increase across the period. Peaks occur at stable configurations (d5, d10).
Melting Point Trends
Melting points indicate the strength of metallic bonding. Stronger bonding occurs when more electrons (from both ns and (n-1)d) participate.
The Mn/Tc Anomaly
Manganese (Mn) and Technetium (Tc) show a sharp dip in melting point. This is because the stable half-filled d5 configuration holds electrons more tightly, making them less available for metallic delocalization.
Oxidation State Landscape
Transition elements exhibit variable oxidation states because both ns and (n-1)d electrons participate in bonding. The number of states increases to a maximum at Manganese, then decreases.
| Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn |
|---|---|---|---|---|---|---|---|---|---|
+7 |
|||||||||
+6 |
+6 |
+6 |
|||||||
+5 |
+5 |
+5 |
|||||||
+4 |
+4 |
+4 |
+4 |
+4 |
+4 |
+4 |
|||
+3 |
+3 |
+3 |
+3 |
+3 |
+3 |
+3 |
+3 |
||
+2 |
+2 |
+2 |
+2 |
+2 |
+2 |
+2 |
+2 |
+2 |
|
+1 |
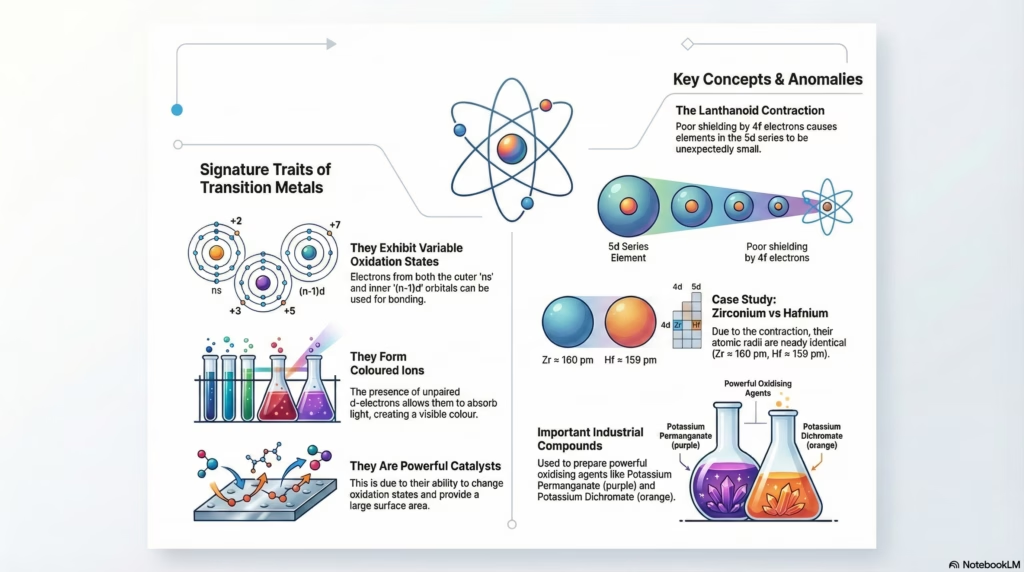
Industrial Preparation Pathways
Preparation of Potassium Dichromate
From Chromite OreFusion
Chromite ore + Na₂CO₃ + Air
(Sodium Chromate – Yellow)
Acidification
Filter & treat with H₂SO₄
(Sodium Dichromate – Orange)
Crystallization
Treat with KCl
(Crystals separate out)
Preparation of Potassium Permanganate
From PyrolusiteOxidative Fusion
MnO₂ + KOH + O₂/KNO₃
(Potassium Manganate – Green)
Electrolytic Oxidation
In Alkaline solution
(Permanganate Ion – Purple)
Reaction Profiles
Dichromate Reactions
Cr₂O₇²⁻ + 14H⁺ + 6e⁻ → 2Cr³⁺ + 7H₂O
Permanganate Reactions
Volumetric Analysis: Redox Titrations
High YieldPotassium Permanganate acts as a self-indicator in acidic medium. Here are the specific half-reactions you must know for titrations.
Green Fe²⁺ turns to Yellow Fe³⁺.
Slow reaction initially; autocatalyzed by Mn²⁺.
Forms Iodine (Purple/Brown in solution).
Sulphite oxidizes to Sulphate.
Master Equation (Acidic): MnO₄⁻ + 8H⁺ + 5e⁻ → Mn²⁺ + 4H₂O
Why do Transition Metals form Complexes?
Transition metals are unique in their ability to form a vast number of complex compounds (like [Fe(CN)₆]³⁻, [Cu(NH₃)₄]²⁺). This property drives biological systems (Hemoglobin) and industrial catalysis.
- Small Size & High Charge: High ionic charge density attracts ligands strongly.
- Availability of d-Orbitals: Empty (n-1)d orbitals of appropriate energy can accept lone pairs from ligands.
The Copper Anomaly (E° Values)
Why is E°(Cu²⁺/Cu) Positive (+0.34V)?
Unlike other 3d metals which have negative E° values (meaning they release H₂ from acids), Copper has a positive E°. This means Copper is less reactive than Hydrogen.
The stability of an ion in solution depends on the energy balance:
Energy Required (Sublimation + Ionization) vs Energy Released (Hydration).
For Copper, the high energy required to transform Cu(s) to Cu²⁺(g) is not fully compensated by its Hydration Enthalpy.
Sublimation + IE₁ + IE₂
Hydration Enthalpy
Visual representation of energy imbalance for Cu
Formation of Colored Ions
Why are some ions colored while others are white?
The d-d Transition Mechanism
In the presence of ligands (like water), the degenerate d-orbitals split into two energy levels (t2g and eg).
When white light falls on the ion, electrons absorb specific wavelengths to jump from the lower to higher d-orbital. The transmitted light is the complementary color we see.
Condition for Color:
Presence of at least one unpaired d-electron.
Ions with d0 or d10 are colorless (e.g., Sc3+, Zn2+).
Purple Solution
d-d Transition possible
Absorbs in visible region
Magnetic Moment (Spin Only):
1.73 B.M.
Structures of Oxoanions
Chromate Ion (CrO₄²⁻)
Tetrahedral Geometry
Yellow Color
Dichromate Ion (Cr₂O₇²⁻)
Two tetrahedra sharing one oxygen at 126° angle.
Orange Color
Permanganate Ion (MnO₄⁻)
Tetrahedral Geometry (π-bonding involved).
Intense Purple
Why are they Catalysts?
Reason 1: Variable Oxidation States
Transition metals can form unstable intermediates by changing oxidation states, lowering activation energy.
Ex: V₂O₅ in Contact Process (V⁵⁺ ↔ V⁴⁺)
Reason 2: Large Surface Area
Provide surface for reactants to adsorb and weaken bonds.
Ex: Finely divided Iron in Haber Process
Alloy Formation
Transition metals have very similar atomic radii (within 15%). Atoms of one metal can easily replace atoms of another in the crystal lattice.
The Lanthanoid Contraction
When moving from the 4d series (Yttrium to Cadmium) to the 5d series (Lanthanum to Mercury), we expect a size increase due to the new shell. This does not happen.
The filling of 14 electrons in the inner 4f orbitals occurs before the 5d series. 4f electrons have a diffuse shape and shield the nucleus poorly. This results in a higher effective nuclear charge pulling the outer electrons inward.
Result: The atomic radii of 4d and 5d metals are nearly identical (e.g., Zr ≈ 160 pm, Hf ≈ 159 pm). This makes separation extremely difficult.
Similarity in size between Zr and Hf is due to Lanthanoid Contraction.
The Inner Transition Matrix
Comparison between 4f (Lanthanoids) and 5f (Actinoids) series. This differentiation is critical for board exams.
High-Yield Q&A
Transition elements are defined as having incompletely filled d-orbitals in their ground state or common oxidation states.
Zn, Cd, and Hg have fully filled d-orbitals (n-1)d10 in both their ground state and stable ions (e.g., Zn2+ is 3d10). Thus, they do not fit the definition.
Scandium (Z=21) has the config [Ar] 3d1 4s2. Sc3+ loses all 3 valence electrons, resulting in [Ar] 3d0.
Since there are zero unpaired electrons, the magnetic moment is 0 B.M. It is diamagnetic.
Cr2+ to Cr3+: Cr2+ (d4) wants to lose an electron to become Cr3+ (d3). The d3 configuration is highly stable in aqueous solutions because it has a half-filled t2g level. Thus, it reduces others and oxidizes itself.
Mn3+ to Mn2+: Mn3+ (d4) wants to gain an electron to become Mn2+ (d5). The d5 configuration is a stable half-filled subshell. Thus, it oxidizes others and reduces itself.
Conceptual Deep Dive
Addressing the most common points of confusion in exams.
Why is the E° value for Mn³⁺/Mn²⁺ couple much more positive than for Cr³⁺/Cr²⁺?
A large positive E° value indicates a strong tendency to get reduced. Mn³⁺ (d⁴) is desperate to become Mn²⁺ (d⁵) because d⁵ is a half-filled, stable configuration. In contrast, Cr³⁺ (d³) is already very stable in aqueous medium (t₂g³), so it has less tendency to accept an electron compared to Mn³⁺.
Why is Cu⁺ unstable in aqueous solution?
Although Cu⁺ has a stable d¹⁰ configuration, it is unstable in water and disproportionates:
2Cu⁺(aq) → Cu²⁺(aq) + Cu(s).
This happens because the Hydration Enthalpy of Cu²⁺ is much higher (more negative) than Cu⁺ due to its smaller size and higher charge. This energy release compensates for the 2nd ionization enthalpy of Copper.
What are Interstitial Compounds?
Transition metals have crystal lattices with empty spaces (voids). Small atoms like H, C, or N get trapped here.
Key Properties:
- High melting points (higher than pure metal).
- Very hard (some approach diamond hardness).
- Retain metallic conductivity.
- Chemically inert.
Why do transition metals exhibit variable oxidation states?
This is due to the very small energy gap between the (n-1)d and ns orbitals. Electrons from both shells can participate in bonding.
However, oxidation states differ by unity (e.g., Fe²⁺, Fe³⁺) unlike non-transition elements which differ by 2 units (e.g., Pb²⁺, Pb⁴⁺) due to the inert pair effect.
Unit Summary & Outcomes
You have navigated the complexities of d and f block elements. The key takeaways for your board exams are:
Master the irregularities in Ionization Enthalpy and Atomic Radii, especially around Zn and Mn.
Always check for unpaired electrons. No unpaired e⁻ = Colorless & Diamagnetic.
KMnO₄ and K₂Cr₂O₄ are your go-to oxidizers. Remember their pH-dependent products.
Class 12 Chemistry Unit 3: Chemical Kinetics – Practice Questions Quiz (2025-26)
Thermodynamics predicts if a reaction occurs, but Chemical Kinetics determines the speed. For Class XII students tackling the 2025-26 syllabus, mastering the “time” variable in chemistry is required for solving numerical problems and interpreting rate graphs correctly.
This guide breaks down the mathematics of reaction rates, comparing Average and Instantaneous speeds while distinguishing experimental Order from theoretical Molecularity. From deriving Integrated Rate Equations for Zero and First Order reactions to calculating Activation Energy using the Arrhenius formula, these sections provide the specific tools needed for board exams.
Explore how concentration, temperature, and catalysts dictate reaction velocity through interactive visualizers designed to clarify the mechanics behind the math.
Rezyo.in
Class XII Unit 3: Chemical Kinetics
Chemical Kinetics: The Dimension of Time
Thermodynamics predicts if a reaction is feasible, but Kinetics defines the speed. This guide covers the complete 2025-26 syllabus for Unit 3, identifying how concentration, temperature, and catalysts dictate the pace of chemical change.
Knowledge Check
Pop-out quiz with timer to test your speed.
Speed & Accuracy Challenge
Ready to test your mastery of Chemical Kinetics? You have 20 questions. The clock starts when you click below.
Rate Concepts & Stoichiometry
Average Rate
Measured over a time interval. Ignores rate fluctuations.
Instantaneous Rate
Rate at a specific moment (\(\Delta t \to 0\)). The slope of the tangent.
Stoichiometric Normalization
For \( aA + bB \to cC \), the unique rate of reaction is found by dividing the rate of change of any species by its stoichiometric coefficient:
Rate = \( -\frac{1}{a}\frac{d[A]}{dt} = -\frac{1}{b}\frac{d[B]}{dt} = \frac{1}{c}\frac{d[C]}{dt} \)
Concept: Pseudo First Order Reaction
A reaction that is theoretically higher order but behaves as first order because one reactant is present in large excess.
Example: Acid hydrolysis of ethyl acetate. Water is in excess, so its concentration remains effectively constant.
\( Rate = k'[Ester][H_2O] \approx k[Ester] \) where \( k = k'[H_2O] \).
Decay Curves
Visualizing Concentration [R] vs Time (t)
Comparison Logic
Rate is constant. Concentration drops linearly. Slope = \(-k\).
\( [R] = -kt + [R]_0 \)
Rate decreases as conc. drops. Exponential decay. Constant Half-Life.
\( \ln[R] = -kt + \ln[R]_0 \)
Half-Life Dynamics
Zero Order: Half-life decreases as concentration drops.
First Order: Half-life remains constant regardless of concentration.
Select a reaction order above.
Arrhenius Equation & Temperature
The Arrhenius equation relates rate constant (\(k\)) to temperature (\(T\)).
\( \ln k = -\frac{E_a}{R}\frac{1}{T} + \ln A \)
Plot of \(\ln k\) vs \(1/T\) (Linear)
Key Parameters
-
Slope (\(m\)): Represents \( -E_a/R \). A steeper slope means higher Activation Energy (\(E_a\)).
-
Intercept (\(c\)): Represents \( \ln A \). \(A\) is the Frequency Factor.
Temperature Coefficient (\(\mu\))
For most reactions, rate constant nearly doubles for a 10° rise in temperature.
\( \mu = \frac{k_{T+10}}{k_T} \approx 2 \) to \( 3 \).
Collision Theory & Catalysis
Reactions occur when molecules collide. However, not all collisions lead to products. Two barriers must be overcome:
- Energy Barrier: Kinetic energy \(\ge\) Activation Energy (\(E_a\)).
- Orientation Barrier: Molecules must align correctly (Steric factor \(P\)).
Mathematical Form
- \(Z_{AB}\): Collision Frequency
- \(P\): Probability/Steric Factor
- \(e^{-E_a/RT}\): Fraction of energetic collisions
Effect of Catalyst
A catalyst provides an alternate pathway with lower \(E_a\). It does not change the Enthalpy (\(\Delta H\)) or Gibbs Energy (\(\Delta G\)) of the reaction. It simply speeds up the attainment of equilibrium.
Numerical Cheat Sheet
Unit of k: \( s^{-1} \)
Independent of concentration
T in Kelvin, R = 8.314
Unit of k: \( mol L^{-1} s^{-1} \)
Conceptual Doubts (FAQ)
Q: Why does a 10° rise in temperature nearly double the rate?
Collision frequency (\(Z\)) only increases by ~3%. The primary reason is the exponential increase in the fraction of effective collisions. A small temperature rise pushes significantly more molecules over the Activation Energy (\(E_a\)) barrier.
Q: Can Molecularity be zero or fractional?
No. Molecularity refers to the count of reacting species colliding simultaneously in an elementary step. You cannot have zero molecules colliding, nor half a molecule. It must be a positive integer.
Q: Do Zero Order reactions go to completion?
Yes. Unlike First Order reactions (which theoretically take infinite time), Zero Order reactions consume reactants at a constant rate and reach completion in finite time: \( t_{completion} = [R]_0 / k \).
Q: Does a catalyst change the Equilibrium Constant (Kc)?
No. A catalyst lowers the activation energy for both forward and reverse reactions equally. It speeds up the attainment of equilibrium but does not shift the position of equilibrium or change \(\Delta G\).
Q: What is the unit of k for an \(n^{th}\) order reaction?
The general formula is \( (mol \ L^{-1})^{1-n} \ s^{-1} \).
For \(n=0\): \(mol \ L^{-1} \ s^{-1}\).
For \(n=1\): \(s^{-1}\).
For \(n=2\): \(mol^{-1} \ L \ s^{-1}\).
Chapter Summary
We have explored the dynamics of chemical change. From defining rates to analyzing the temperature dependence via Arrhenius, you now possess the tools to calculate when a reaction completes. Remember: Order is experimental, Molecularity is theoretical, and Temperature increases rate by increasing effective collisions, not just total collisions.

Class 12 Electrochemistry Practice Exam: Nernst Calculator, Logic Lab & Board Simulator
Electrochemistry links spontaneous chemical reactions to electrical output. This Unit 2 guide moves beyond static notes, offering a hands-on learning dashboard. Students can gauge their readiness with a timed board-exam simulator, solve numericals instantly using dedicated calculators, and predict electrolysis products through logic trees. Whether reviewing the Nernst equation or comparing commercial batteries, these tools support practical understanding and exam performance.
Rezyo.in
Class 12 • Electrochemistry
Ready to test your
Electrochemistry concepts?
Time Limit
25 Minutes
Questions
25 MCQs
Target
Board Level
Student Candidate
ID: 2026-CHEM-XII
Question Palette
Exam Submitted!
Here is your detailed performance analysis.
Total Score
0/0
Accuracy
0%
Time Taken
0m
Detailed Solutions
Battery Technology Hub
Dry Cell
Primary- Anode: Zinc Cup
- Cathode: Graphite Rod
- Electrolyte: NH₄Cl + ZnCl₂ paste
- Volt: ~1.5 V
Mercury Cell
Primary- Anode: Zn-Hg Amalgam
- Cathode: HgO + Carbon
- Volt: 1.35 V (Constant)
- Use: Watches, Hearing aids
Lead Storage
Secondary- Anode: Spongy Lead (Pb)
- Cathode: PbO₂ Grid
- Electrolyte: 38% H₂SO₄
- Recharge: PbSO₄ dissolves back
H₂-O₂ Fuel Cell
Efficiency- Anode: H₂ fed (Oxidation)
- Cathode: O₂ fed (Reduction)
- Byproduct: Pure Water
- Efficiency: η = ΔG/ΔH (~70%)
Conductivity Measurement
To find conductivity (κ), we first measure Resistance (R) using a Wheatstone Bridge with AC current (to prevent electrolysis).
(Rx)
Electrolysis Logic Lab
Predict the anode product using step-by-step reasoning.
Λm vs √C Trends
Nernst Calc
Kohlrausch Calc (Λ°m)
Can We Store It?
Check if a solution can be kept in a metal container.
Faraday’s 2nd Law: Series Connection
Charge (Q) is same for all.
Mass ∝ Equivalent Weight
m₁/m₂ = E₁/E₂
Corrosion Prevention Strategies
Sacrificial Protection
Coating with a more reactive metal (e.g., Zinc on Iron). Zinc oxidizes (E° = -0.76V) instead of Iron (-0.44V). This is Galvanization.
Cathodic Protection
Connecting iron pipes to Mg or Zn blocks. The pipe becomes the cathode (protected) and the block becomes the anode (sacrificed).
Quick Revision Formulae
Conceptual FAQs
Why does conductivity decrease with dilution?
Can we store CuSO₄ in Zinc pot?

Class 12 Electrochemistry Notes & Calculator | Unit 2 Nernst Equation, Conductance & Batteries Guide
Electrochemistry connects spontaneous chemical reactions to electrical energy production. This Unit 2 syllabus guide simplifies electrolytic conductance, the Nernst equation, and the working principles of commercial batteries.
Students can compute cell potentials directly with the built-in calculator, track ion migration through visual animations, and test their grasp of Faraday’s Laws with the integrated quiz. From the standard hydrogen electrode to the chemistry of rusting, these notes prioritize exam-focused concepts and numerical problem-solving.
Electrochemistry:
From Ions to Energy
A comprehensive guide to understanding how chemical energy powers our world. Covers conduction, Nernst equation, commercial batteries, and corrosion.
01 Electrolytic Conductance
Unlike metallic wires where electrons flow through a lattice, electrolytic conduction relies on the physical movement of ions. This creates a unique relationship between concentration and resistance.
κ = G × G*
Where G is Conductance and G* is the Cell Constant (l/A)
Λm = (κ × 1000) / C
Units: S cm2 mol-1
Visualizing Dilution Effect
Animation: As water is added (Dilution), ions move apart.
κ decreases (fewer ions/vol), but Λm increases (higher mobility).
Factors Affecting Electrolytic Conductance
1. Nature of Electrolyte
Strong electrolytes dissociate completely (high conductance). Weak electrolytes dissociate partially (low conductance).
2. Size of Ions & Solvation
Larger ions (due to hydration) move slower. Example: Li+ is heavily hydrated and moves slower than Cs+.
3. Nature of Solvent & Viscosity
Polar solvents favor ionization. Higher viscosity resists ion flow, lowering conductance.
4. Temperature
Unlike metals, electrolytic conductance increases with temperature as kinetic energy increases and interionic attractions weaken.
Strong vs. Weak Electrolytes
| Feature | Strong Electrolyte (e.g., KCl) | Weak Electrolyte (e.g., CH3COOH) |
|---|---|---|
| Dissociation | Complete at all concentrations. | Partial. Increases with dilution. |
| Conductivity Rise | Gradual linear increase. | Steep, exponential increase near zero conc. |
| Reason | Reduced interionic drag. | Increase in number of ions (Ostwald Law). |
| Limiting Value | Found by extrapolation of graph. | Found using Kohlrausch Law. |
02 Conductivity Trends
03 Kohlrausch’s Law of Independent Migration
At infinite dilution, every ion makes a definite contribution towards the molar conductivity of the electrolyte, regardless of the nature of the other ion it is associated with.
Application: This law is critical for calculating the limiting molar conductivity (Λ°m) for weak electrolytes, which cannot be determined graphically.
04 Nernst Equation & Gibbs Energy
The Nernst equation links the potential of a cell to the concentration of species involved. It bridges thermodynamics and electrochemistry.
Ecell = E°cell – (0.0591 / n) log Q
ΔG° = -nFE°cell
n = moles of electrons, F = 96500 C
Equilibrium Constant & Cell Potential
When an electrochemical cell reaches equilibrium, the reaction stops flowing. At this stage, Ecell = 0 and the reaction quotient Q becomes Kc (Equilibrium Constant).
05 Nernst Equation Calculator
Calculate the cell potential ($E_{cell}$) for a reaction at 298K.
Cell Potential ($E_{cell}$)
06 Electrochemical Series Explorer
The Reference: Standard Hydrogen Electrode (SHE)
Since individual half-cell potentials cannot be measured, the SHE is the universal reference.
- Setup: Platinum foil coated with platinum black dipped in 1M H⁺ solution.
- Convention: Its potential is arbitrarily fixed at 0.00 V at all temperatures.
- Role: Acts as Anode or Cathode depending on the other half-cell.
07 Faraday’s Laws Calculator
First Law: The mass of substance deposited is directly proportional to the charge passed. (m = ZIt).
Mass Deposited
Formula Used: m = (M × I × t) / (n × 96500)
08 Deep Dive: Electrolysis Products
Predicting products of electrolysis requires analyzing Standard Potentials and considering Overpotential.
Case A: Molten NaCl
Only two ions exist: Na+ and Cl–.
- Cathode: Na+ + e– → Na(s)
- Anode: Cl– → ½Cl2 + e–
Case B: Aqueous NaCl (Brine)
Competition with Water molecules.
-
At Cathode:
H+ discharges (E°=0.0V) instead of Na+ (E°=-2.71V).
Product: H2 gas. -
At Anode:
Cl– oxidizes to Cl2. Oxygen is expected (lower E°), but Overpotential makes Cl2 easier to form.
Product: Cl2 gas.
Comparison: The Two Cell Types
| Parameter | Electrochemical (Galvanic) Cell | Electrolytic Cell |
|---|---|---|
| Energy Conversion | Chemical Energy → Electrical Energy | Electrical Energy → Chemical Energy |
| Spontaneity | Spontaneous (ΔG < 0) | Non-Spontaneous (ΔG > 0) |
| Anode Charge | Negative (-) | Positive (+) |
| Cathode Charge | Positive (+) | Negative (-) |
09 Commercial Batteries
Dry Cell (Leclanché)
Primary- • Anode: Zinc Container
- • Cathode: Carbon (Graphite) rod
- • Voltage: ~1.5 V (Non-constant)
Mercury Cell
Primary- • Anode: Zn-Hg Amalgam
- • Cathode: Paste of HgO + C
- • Voltage: 1.35 V (Constant)
- • Key Fact: No ions in overall reaction.
Lead Storage Battery
Secondary- • Anode: Pb (Lead)
- • Cathode: PbO2
- • Electrolyte: 38% H2SO4
Nickel-Cadmium (NiCd)
Secondary- • Anode: Cadmium (Cd)
- • Cathode: Ni(OH)3
- • Voltage: ~1.4 V
- • Life: Longer life than Lead storage.
Fuel Cell Efficiency Focus
Fuel cells, like the H2-O2 cell used in the Apollo space program, convert energy of combustion directly into electricity.
10 Corrosion: Electrochemical Theory
Rusting of iron is essentially setting up of a tiny electrochemical cell on the surface of the metal.
Prevention: Barrier protection (painting), Galvanization (coating with Zinc), or Cathodic Protection (connecting to Mg or Zn).
11 Quick Check Quiz
1. For a spontaneous reaction, ΔG must be:
2. In an Electrolytic cell, the Anode is:
3. The unit of Molar Conductivity is:
12 Exam Focus Q&A & Doubts
Q1: Why does conductivity decrease but molar conductivity increase with dilution?
+Think about ions per unit volume vs total volume.
Conductivity (κ) decreases because the number of ions per unit volume decreases on dilution.
Molar Conductivity (Λm) increases because the volume containing one mole of electrolyte increases significantly, outweighing the decrease in κ.
What is the exact function of a Salt Bridge?
1. Completes the electrical circuit.
2. Maintains electrical neutrality by allowing flow of ions.
Difference between EMF and Potential Difference?
- EMF: Potential diff when no current flows (Max voltage).
- Potential Diff: Potential diff when current is flowing (Less than EMF).
⚡ Key Formula Cheat Sheet
G = 1/R = κ (A/l)
Cell Constant (G*) = l/A
α = Λm / Λ°m
ΔG° = -nFE°cell
Glossary of Terms
Conclusion
Electrochemistry connects chemical reactions with electricity. This master guide has covered everything from fundamental conductance to complex commercial batteries. Remember to practice the numericals using the provided calculators and review the cheat sheet before exams.
© 2026 Rezyo.in. All rights reserved.

Class 12 Chemistry Unit 1: Solutions – Practice Questions Quiz (2025-26)
Physical Chemistry often feels abstract until you see how the variables interact. Unit 1: Solutions establishes the mathematical framework for the Class 12 syllabus, linking concentration terms to observable changes in vapor pressure and boiling points. This page moves beyond static textbook definitions to offer a hands-on learning experience.
Here, you will find a dynamic dashboard to test your grasp of Molarity, Molality, and Henry’s Law in real-time. We break down the thermodynamics behind Ideal and Non-Ideal solutions using comparison matrices and deviation logic trees, allowing you to predict positive or negative deviations without rote memorization. From visualizing surface barriers in vapor pressure to calculating the Van’t Hoff factor for complex ions, this resource aligns strictly with the 2025-26 academic standards to prepare you for board exams and competitive tests.
Rezyo.in
Class XII Chemistry Unit 1 • 2025-26
Select a filter below to start practicing concepts.
Thermodynamics of Solution Mixing
Solubility Dynamics
Solid in Liquid
- Effect of Pressure Negligible
- Temp (Endothermic $\Delta H > 0$) Increases
- Temp (Exothermic $\Delta H < 0$) Decreases
Gas in Liquid
- Effect of Pressure Increases (Henry’s Law)
- Effect of Temp Decreases
- Henry’s Constant ($K_H$) Higher $K_H$ = Lower Solubility
Azeotrope Classification Hub
Minimum Boiling Azeotrope
Deviation: Large Positive
Example: Ethanol (95.5%) + Water (4.5%)
BP: 351.15 K (Lower than water 373K)
Maximum Boiling Azeotrope
Deviation: Large Negative
Example: Nitric Acid (68%) + Water (32%)
BP: 393.5 K (Higher than water 373K)
Visual Concept: Surface Barrier Effect
Why does adding a non-volatile solute lower vapour pressure?
Raoult’s Law Deviations
Deviation Logic Tree
Solute (A) & Solvent (B)
Conceptual Doubts (FAQ)
Q: Why does adding salt to ice melt it (Depression in Freezing Point)?
Adding a non-volatile solute (salt) lowers the freezing point of the solvent (water). If the ambient temperature is higher than this new, lowered freezing point (e.g., -5°C), the ice cannot exist as a solid and melts into liquid water.
Q: Why are aquatic species more comfortable in cold water?
This relates to Henry’s Law. The value of Henry’s constant ($K_H$) increases with temperature. Since solubility is inversely proportional to $K_H$, the solubility of oxygen decreases as water gets warmer.
Q: What is the medical condition “Edema”?
Excess salt intake increases ion concentration in tissue fluids. Water moves out of cells via osmosis to balance this, causing water retention and puffiness known as Edema.
Chapter Summary & Outcomes
You have navigated the complexities of Solution Chemistry. From mastering concentration units to applying the Van’t Hoff factor for ionic compounds, these concepts form the bedrock of Physical Chemistry.

Class 12 Chemistry Unit 7: Alcohols, Phenols & Ethers | Notes, Mechanisms & Reaction Maps (2025-26)
Organic compounds containing Carbon-Oxygen bonds form a massive part of industrial and biological chemistry. Unit 7 focuses on three specific classes: Alcohols, Phenols, and Ethers.
This guide breaks down their structural differences, nomenclature rules, and preparation methods, such as Hydroboration-Oxidation and the Cumene process. You will study specific reaction mechanisms, including the acid-catalyzed hydration of ethene, and learn to distinguish between primary, secondary, and tertiary alcohols using the Lucas Test.
Try Practice Questions and Answers with Hints
From understanding the acidity of phenols to mastering the cleavage of ethers with hydrogen iodide, these resources cover the essential concepts required for board exams and competitive entrance tests.
Unit 7: Alcohols, Phenols, and Ethers
Class XII Chemistry (2025-26 Session). Focusing on Competency-Based Assessment, Molecular Classification, and Reaction Mechanisms.
2025-26 Curricular Updates
The current session emphasizes competency-based education. Students must apply concepts rather than rely on rote memorization.
CBSE Focus
- Green Chemistry context.
- Mechanism identification.
- Material science applications.
ISC Focus
- Dow’s process specifics.
- Aryl ether substitution.
- Detailed mechanism coverage.
WBCHSE Focus
- Semester-based integration.
- Acidic nature of phenol.
- Traditional functional tests.
Nomenclature Pitfalls
Confusion often arises between Common and IUPAC names. Below are high-frequency exam compounds.
| Structure | Common Name | IUPAC Name |
|---|---|---|
| HO-CH2-CH2-OH | Ethylene Glycol | Ethane-1,2-diol |
| CH2(OH)-CH(OH)-CH2(OH) | Glycerol | Propane-1,2,3-triol |
| OH-C6H4-CH3 (ortho) | o-Cresol | 2-Methylphenol |
| C6H5-O-CH3 | Anisole | Methoxybenzene |
| C6H5-O-C2H5 | Phenetole | Ethoxybenzene |
Structural Geometry & Hybridization
| Molecule | Angle | Reason |
|---|---|---|
| Methanol | 108.9° | Lone pair repulsion. |
| Phenol | 109.0° | sp2 carbon effect. |
| Ether | 111.7° | Steric repulsion. |
Physical Properties
Boiling Point Trends
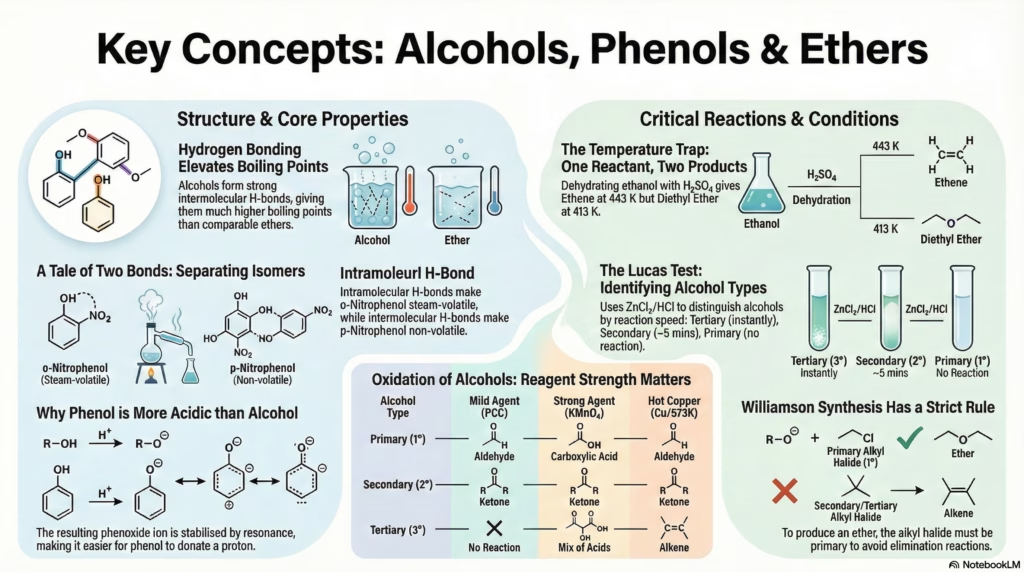
Alcohols have significantly higher boiling points than hydrocarbons and ethers of comparable molecular mass due to Intermolecular Hydrogen Bonding.
Solubility Dynamics
Lower Alcohols
Highly soluble in water due to H-bonds with water molecules.
Effect of Mass
Solubility decreases as the hydrophobic alkyl group size increases.
Separation: o-Nitrophenol vs p-Nitrophenol
Ortho-Nitrophenol
Volatile (Steam Distillable)
Due to Intramolecular H-Bonding (within the same molecule), it does not associate strongly with other molecules, lowering its boiling point.
Para-Nitrophenol
Non-Volatile (Remains in flask)
Due to Intermolecular H-Bonding (between different molecules), it forms associated aggregates, significantly raising the boiling point.
Mechanism Explorer
InteractiveTopic: Acid Catalysed Hydration of Ethene to Ethanol.
Step 1: Protonation
Hydronium ion transfers a proton to the double bond.
Step 2: Nucleophilic Attack
Water attacks the carbocation.
Step 3: Deprotonation
Loss of proton regenerates catalyst.
Preparation Methods
1. From Cumene (Commercial)
High YieldMost phenols produced via this method; Acetone is a valuable by-product.
2. Hydroboration-Oxidation
Alkene + (BH3)2 → Alcohol
Rule: Follows Anti-Markovnikov addition.
Commercial & Green Chemistry
Methanol
CO + 2H2 → CH3OH. Highly toxic (causes blindness).
Ethanol
Fermentation of molasses. Denatured with CuSO4/Pyridine.
Green Context
Ethanol is used as a bio-solvent replacing toxic chlorides, and as a fuel blend (Gasohol) to reduce emissions.
Reaction Mechanisms
Grignard Synthesis
| Reactant | Result |
|---|---|
| HCHO | Primary Alcohol |
| RCHO | Secondary Alcohol |
| RCOR | Tertiary Alcohol |
Williamson Ether Synthesis
R-X + R’-ONa → R-O-R’. Constraint: R-X must be Primary.
Tertiary Halide causes Elimination (Alkene) instead of Substitution.
Acidity & Modifiers
EWG (NO2): Increases Acidity (Stabilizes anion).
EDG (CH3): Decreases Acidity (Destabilizes anion).
Ether Cleavage (with HI)
Mechanism: SN2. I– attacks smaller group.
Mechanism: SN1. I– attacks Tertiary C (Stable Carbocation).
Ether Cleavage Decision Tree
Reacting R-O-R’ with HI. Where does the Iodide go?
Iodide attacks Tertiary Carbon.
Iodide attacks Smaller/Less hindered group.
The Temperature Trap: 413K vs 443K
The dehydration of alcohols with concentrated H2SO4 yields completely different products based on the temperature.
At 443 K (170°C)
C2H5OH → CH2=CH2 + H2O
Mechanism: Elimination.
Product: Alkene (Ethene).
Thermodynamically favored at higher temperatures (entropy increases).
At 413 K (140°C)
2 C2H5OH → C2H5-O-C2H5
Mechanism: Nucleophilic Substitution (SN2).
Product: Ether (Diethyl Ether).
Requires excess alcohol.
Synthesis of Aspirin (Acetylsalicylic Acid)
Produced by the acetylation of Salicylic acid using Acetic Anhydride in the presence of acid catalyst.
- Reactant: Salicylic Acid (2-Hydroxybenzoic acid)
- Reagent: Acetic Anhydride + Conc H2SO4
- Product: Aspirin (Analgesic & Antipyretic)
- By-product: Acetic Acid (Vinegar smell)
Resonance in Phenol & Phenoxide
Why is Phenol acidic and Ortho/Para directing?
1. Acidic Nature
The Phenoxide ion formed after losing H+ is resonance stabilized. The negative charge is delocalized over the benzene ring.
Ortho → Para → Ortho positions.
Comparison: Alkoxide ion (R-O–) has no resonance, making alcohols weaker acids than phenol.
2. Ortho/Para Directing
The lone pair on Oxygen is pushed into the ring (+R Effect), increasing electron density.
Ortho (2,6) and Para (4) positions.
Electrophiles (E+) attack these electron-rich positions.
Key Conversion Pathways
Convert Ethanol to Acetone (Propanone)
Convert Phenol to Aspirin
The Haloform (Iodoform) Test
Key Identification Test
Reagents: NaOH + I2 (or NaOI).
Positive Result: Formation of Yellow Precipitate (CHI3).
Condition: The molecule must contain the CH3-CH(OH)- group (methyl carbinol) or CH3-C=O group.
Oxidation Pathways
| Type | Mild (PCC) | Strong (KMnO4) | Cu / 573K |
|---|---|---|---|
| 1° | Aldehyde | Acid | Aldehyde |
| 2° | Ketone | Ketone | Ketone |
| 3° | None | Mix of Acids | Alkene |
Electrophilic Substitution
Phenol (OH is Activator)
- Br2 / CS2: Mono-substituted (o/p-Bromophenol).
- Br2 Water: Poly-substituted (2,4,6-Tribromophenol – White PPT).
- Conc. HNO3: Picric Acid (2,4,6-Trinitrophenol).
Anisole (OR is Activator)
- Friedel-Crafts: CH3Cl/AlCl3 gives o/p-Methoxy toluene.
- Nitration: Gives mixture of o/p-Nitroanisole.
Named Reactions
Reimer-Tiemann
Phenol + CHCl3 + NaOH → Salicylaldehyde.
Intermediate: Dichlorocarbene (:CCl2).
Kolbe’s Reaction
Sod. Phenoxide + CO2 → Salicylic Acid.
Phenol Conversion Roadmap
Interactive Lab Bench
Select a test and a sample to see the virtual result.
Ready
Reference Material
Distinction Table
| Test | Alcohol | Phenol |
|---|---|---|
| Litmus | Neutral | Blue → Red |
| FeCl3 | No Rxn | Violet Color |
| Br2 Water | No Rxn | White PPT |
Reagent Cheat Sheet
1° Alc → Aldehyde
Acid → Alcohol
Phenol → Benzene
Alc → Alkene
Alc → Ether
Phenol → Quinone
Flashcards
Tap to flipQuiz
1. Reagent to convert Phenol to Benzene?
2. Electrophile in Reimer-Tiemann?
3. Product of 3° Alcohol + Cu/573K?
Frequently Asked Questions
Q: Why is Phenol more acidic than Ethanol?
A: The phenoxide ion formed after losing a proton is resonance stabilized (negative charge delocalized over the benzene ring). The ethoxide ion is not stabilized and is actually destabilized by the +I effect of the ethyl group.
Q: Why do boiling points of alcohols decrease with branching?
A: Branching makes the molecule more spherical, reducing its surface area. This decreases the Van der Waals forces between molecules, lowering the boiling point.
Q: Why can’t we prepare Anisole from Bromobenzene and Sodium Methoxide?
A: Bromobenzene (Aryl Halide) has a partial double bond character in the C-Br bond due to resonance, making it extremely unreactive towards nucleophilic substitution. The correct method uses Sodium Phenoxide and Methyl Bromide.
Q: Why is sulphuric acid used in esterification?
A: It acts as a dehydrating agent to remove the water formed, shifting the equilibrium to the right (forward direction) to yield more ester, and also acts as a catalyst.
Q: How to distinguish between 1°, 2°, and 3° alcohols?
A: Use the Lucas Test (ZnCl2 + HCl). 3° alcohols react immediately (turbidity), 2° react in ~5 mins, and 1° do not react at room temperature.
Summary & Conclusion
This unit has covered the critical aspects of Alcohols, Phenols, and Ethers, moving from basic nomenclature to complex reaction mechanisms.
Key Takeaways:
- Structure Dictates Property: Hydrogen bonding elevates boiling points and solubility.
- Resonance Rules: The acidity of phenol and its electrophilic substitution patterns are driven by resonance.
- Reagent Specificity: The outcome of reactions (like Oxidation or Dehydration) depends heavily on the specific reagent and conditions (Temperature, Strength).
Mastering these concepts requires understanding the “Why” behind the reactions—steric hindrance, electronic effects, and stability of intermediates—rather than just memorizing equations.
