CBSE Class 12 Amines Notes: Preparation, Reactions, Nomenclature & Mechanisms
Nitrogenous organic compounds function as essential components in synthesis and biology. This study guide for CBSE Class 12 Chemistry Unit 13 examines Amines, categorizing them as primary, secondary, or tertiary derivatives of ammonia. The content maps out core concepts ranging from orbital hybridization and pyramidal geometry to practical laboratory methods like the reduction of nitro compounds and nitriles.
Try our Practice Test
Students will find specific breakdowns of chemical reactions, including the Carbylamine reaction and Hinsberg’s test, alongside mechanism explanations for Hoffmann Bromamide degradation. The notes also address physical properties, such as boiling point variations, and analyze the resonance stability that differentiates aryl amines from alkyl amines. Sections on spectroscopic identification and nitrogen inversion provide advanced context for understanding molecular behavior.
Amines
Comprehensive notes on Structure, Classification, Nomenclature, Preparation, and Properties of Amines for CBSE Class 12.
Contents
1. Introduction
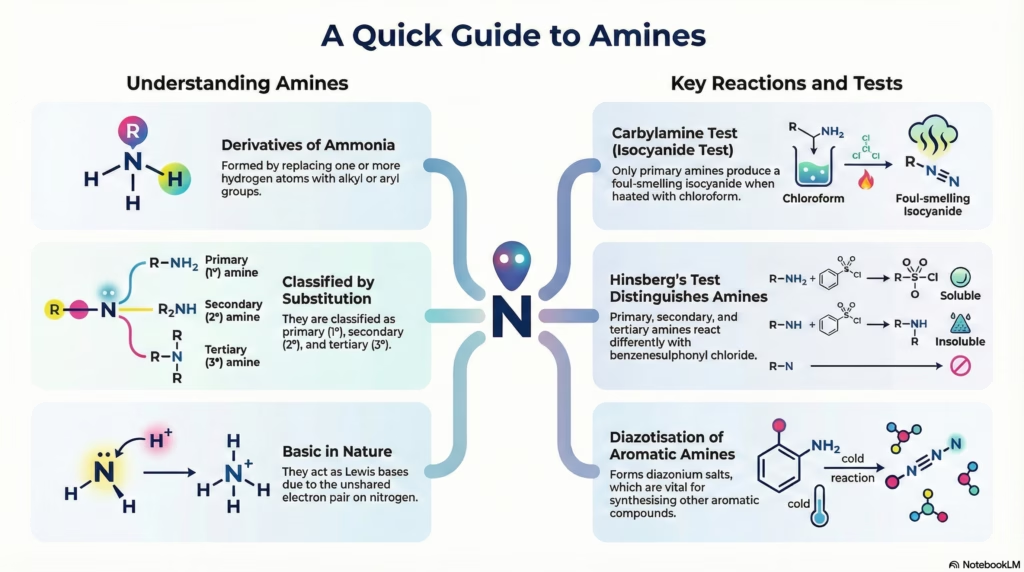
Amines can be considered as derivatives of ammonia (NH3), obtained by replacement of one, two or all the three hydrogen atoms by alkyl and/or aryl groups.
Structure of Amines
Nitrogen has 5 valence electrons. In amines, nitrogen is sp3 hybridised. The shape is pyramidal due to the presence of one lone pair of electrons. The bond angle is slightly less than 109.5° (e.g., 108° in trimethylamine).
2. Classification
One hydrogen atom of ammonia is replaced by an alkyl/aryl group.
Two hydrogen atoms of ammonia are replaced by alkyl/aryl groups.
All three hydrogen atoms of ammonia are replaced by alkyl/aryl groups.
3. Nomenclature
| Structure | Common Name | IUPAC Name |
|---|---|---|
| CH3-NH2 | Methylamine | Methanamine |
| CH3-CH2-NH2 | Ethylamine | Ethanamine |
| CH3-NH-CH3 | Dimethylamine | N-Methylmethanamine |
| C6H5-NH2 | Aniline | Aniline or Benzenamine |
4. Methods of Preparation
1. Reduction of Nitro Compounds
Nitro compounds are reduced to amines by passing hydrogen gas in the presence of finely divided nickel, palladium or platinum and also by reduction with metals in acidic medium.
2. Ammonolysis of Alkyl Halides
An alkyl halide reacts with an ethanolic solution of ammonia to undergo nucleophilic substitution reaction.
R-X + NH3 → R-NH2 → R2NH → R3N → R4N+X–
3. Reduction of Nitriles
Gabriel Phthalimide Synthesis
Important: Only for preparation of primary aliphatic amines. Aromatic primary amines cannot be prepared by this method because aryl halides do not undergo nucleophilic substitution with the anion formed by phthalimide.
Hoffmann Bromamide Degradation
Preparation of primary amines by treating an amide with bromine in an aqueous or ethanolic solution of NaOH. The amine formed contains one carbon less than the parent amide.
5. Physical Properties
Solubility
Lower aliphatic amines are soluble in water because they can form hydrogen bonds with water molecules. Solubility decreases with an increase in molar mass of amines due to an increase in the size of the hydrophobic alkyl part.
Boiling Points
Order of boiling points of isomeric amines:
Primary amines have the highest boiling points due to extensive intermolecular hydrogen bonding.
6. Chemical Reactions
Basic Character of Amines
Amines act as Lewis bases due to the presence of a lone pair of electrons on the nitrogen atom.
Basicity Order in Gaseous Phase:
Tertiary (3°) > Secondary (2°) > Primary (1°) > NH3
Basicity Order in Aqueous Solution (Ethyl group):
2° > 3° > 1° > NH3
(Due to combination of inductive effect, solvation effect, and steric hindrance)
Carbylamine Reaction
Test for Primary Amines only (Aliphatic & Aromatic).
Reaction with Aryl Sulphonyl Chloride (Hinsberg’s Reagent)
Used to distinguish between 1°, 2°, and 3° amines.
- 1° Amine: Forms precipitate soluble in alkali.
- 2° Amine: Forms precipitate insoluble in alkali.
- 3° Amine: Does not react.
7. Electrophilic Substitution
The -NH2 group is ortho and para directing and a powerful activating group.
Bromination
Reacts with bromine water to give 2,4,6-tribromoaniline (White ppt).
Nitration
Direct nitration gives significant amount of meta-derivative along with ortho and para isomers.
Sulphonation
Reacts with H2SO4 to form Zwitter ion (Sulphanilic acid).
8. Mechanisms in Focus
Mechanism: Hoffmann Bromamide Degradation
This reaction involves the migration of an alkyl or aryl group from the carbonyl carbon to the nitrogen atom. The amine formed has one carbon less than the starting amide.
Formation of N-bromoamide
R-CONH2 + Br2 + OH– → R-CONHBr + H2O + Br–
Formation of Acylnitrene Intermediate
Loss of proton from nitrogen by base, followed by loss of bromide ion to form an unstable nitrene species.
Rearrangement (Wolff Rearrangement Analogue)
The alkyl group (R) migrates from Carbon to Nitrogen, forming an Isocyanate (R-N=C=O).
Hydrolysis
R-N=C=O + 2OH– → R-NH2 + CO32-
9. Resonance & Stability
Why is Aniline less basic than Alkylamines?
In aniline, the -NH2 group is attached directly to the benzene ring. The lone pair of electrons on the nitrogen atom enters into conjugation with the benzene ring and is thus less available for protonation.
Resonance Structures of Aniline:
The lone pair delocalizes onto the Ortho and Para positions, creating electron density at these points.
(Neutral)
(Ortho -)
(Para -)
(Ortho -)
(Neutral)
Reactivity Consequence
Since electron density increases at ortho and para positions, electrophiles attack at these locations.
Stability of Anilinium Ion
The anilinium ion formed after accepting a proton has only two Kekule structures and is less stable than the unprotonated aniline (which has 5 resonance structures).
10. Applications & Significance
Synthetic Dyes
Aromatic amines are crucial in the dye industry. Diazonium salts are used to produce Azo dyes (e.g., Methyl Orange, Aniline Yellow) via coupling reactions.
Pharmaceuticals
Amines are biologically active. Sulpha drugs (sulphonamides) derived from benzenesulphonyl chloride are powerful antibacterials. Novocaine is an amine-based anesthetic.
Biological Role
Biologically important amines include Adrenaline and Noradrenaline (neurotransmitters) which regulate blood pressure. Histamine causes allergic reactions.
10. Spectroscopic Identification
Analytical techniques are essential in modern research for identifying amine structures.
Infrared (IR) Spectroscopy
- Region: N-H stretching occurs at 3300–3500 cm-1.
- Primary Amines: Show two bands (symmetric and asymmetric stretching).
- Secondary Amines: Show a single band (weaker).
- Tertiary Amines: Show no absorption in this region (no N-H bond).
NMR Spectroscopy
- Chemical Shift: Protons on the α-carbon are deshielded (2.2–3.0 ppm).
- N-H Protons: Variable chemical shift (0.5–5.0 ppm) due to hydrogen bonding and exchange rate.
- Broadening: Signals often appear broad due to Quadrupole moment of Nitrogen.
11. Nitrogen Inversion
Pyramidal Inversion (Umbrella Flip)
Although nitrogen in amines is sp3 hybridized and chiral when attached to three different groups, individual enantiomers cannot usually be isolated. This is due to rapid pyramidal inversion at room temperature, where the nitrogen atom oscillates through the plane of the substituents.
Note: Quaternary ammonium salts (R4N+) cannot undergo inversion (no lone pair) and thus can be resolved into stable enantiomers if the four groups are different.
12. Study Guide & Quiz
This guide provides a review of key concepts related to amines and diazonium salts. Test your recall with the short-answer quiz below.
Instructions: Click on a question to reveal the answer.
Describe the geometry and orbital hybridization of the nitrogen atom in amines. What is the approximate bond angle and why?
Explain why alkylamines are generally stronger bases than ammonia.
What is the primary limitation of the Gabriel phthalimide synthesis?
How does the Hoffmann bromamide degradation reaction alter the carbon skeleton?
Describe the Carbylamine reaction and its use.
Why is the activating effect of the amino group in aniline often controlled?
Explain why aniline does not undergo Friedel-Crafts reactions.
What are the reaction conditions for the diazotisation of aniline?
What is the key difference between Sandmeyer and Gatterman reactions?
Define a coupling reaction.
13. Essay Questions
1. Basicity Trends
Compare and contrast the basicity of primary, secondary, and tertiary alkylamines in both the gaseous phase and in an aqueous solution. Provide a detailed explanation for the observed trends in each phase, discussing the specific roles of the inductive effect, solvation effect, and steric hindrance.
2. Diazonium Salts Utility
Discuss the synthetic utility of diazonium salts as intermediates in the synthesis of aromatic compounds. Describe at least five distinct types of substitution reactions involving the displacement of the diazonium group, providing the specific reagents required for each transformation.
3. Electrophilic Substitution Challenges
Explain the chemical challenges encountered during the direct electrophilic substitution (specifically nitration and bromination) of aniline. Detail the chemical strategy used to overcome these challenges to produce monosubstituted products, explaining how the protecting group modifies the reactivity of the aromatic ring.
4. Chemical Tests
Describe a series of chemical tests that could be used to definitively distinguish between an unknown primary amine, secondary amine, and tertiary amine. For each test (e.g., Hinsberg’s test, Carbylamine test), explain the reagents used, the expected chemical reaction, and the observable results for each class of amine.
5. Preparation Methods
Elaborate on three different methods for the preparation of primary amines: Reduction of Nitro Compounds, Gabriel Phthalimide Synthesis, and Hoffmann Bromamide Degradation. For each method, detail the starting materials, reagents, and any significant advantages, disadvantages, or unique features (e.g., effect on carbon chain length).
14. Glossary of Key Terms
- Acylation
- A reaction where aliphatic or aromatic primary and secondary amines react with acid chlorides, anhydrides, and esters. It involves the replacement of a hydrogen atom of the -NH2 or >N-H group by an acyl group to form an amide.
- Amine
- An important class of organic compounds derived by replacing one or more hydrogen atoms of an ammonia molecule with alkyl and/or aryl groups.
- Ammonolysis
- The process of cleavage of a C-X (carbon-halogen) bond by an ammonia molecule. It is a nucleophilic substitution reaction where an alkyl or benzyl halide reacts with an ethanolic solution of ammonia to replace the halogen with an amino (-NH2) group.
- Arylamine
- An amine in which the -NH2 group is directly attached to a benzene ring. Aniline (C6H5NH2) is the simplest example.
- Azo Compounds
- Coloured compounds that have an extended conjugate system with two aromatic rings joined through an -N=N- bond. They are used as dyes and are formed via coupling reactions.
- Basic Character of Amines
- The ability of amines to act as Lewis bases due to the unshared pair of electrons on the nitrogen atom. They react with acids to form salts.
- Benzoylation
- A specific type of acylation reaction where an amine reacts with benzoyl chloride (C6H5COCl).
- Carbylamine Reaction
- Also known as the isocyanide test. A reaction where aliphatic and aromatic primary amines are heated with chloroform and ethanolic potassium hydroxide to form foul-smelling substances called isocyanides or carbylamines. It is used as a test for primary amines.
- Coupling Reaction
- A reaction involving the retention of the diazo group, where a diazonium salt reacts with an electron-rich aromatic compound (like phenol or aniline) to form a coloured azo compound. It is an electrophilic substitution reaction.
- Diazonium Salts
- A class of compounds with the general formula R-N2+X–, where R is an aryl group. The N2+ group is called the diazonium group. They are important intermediates in the synthesis of aromatic compounds.
- Diazotisation
- The process of converting a primary aromatic amine into a diazonium salt by reacting it with nitrous acid at low temperatures (273-278 K).
- Gabriel Phthalimide Synthesis
- A method used for the preparation of primary amines. It involves treating phthalimide with ethanolic KOH, followed by heating with an alkyl halide and subsequent alkaline hydrolysis.
- Gatterman Reaction
- A reaction to introduce chlorine or bromine into a benzene ring by treating a diazonium salt solution with the corresponding halogen acid in the presence of copper powder.
- Hinsberg’s Reagent
- The common name for benzenesulphonyl chloride (C6H5SO2Cl). It is used to distinguish between primary, secondary, and tertiary amines.
- Hoffmann Bromamide Degradation
- A method for preparing primary amines by treating an amide with bromine in an aqueous or ethanolic solution of sodium hydroxide. The resulting amine contains one carbon atom less than the parent amide.
- pKb
- A measure of the basicity of a substance; defined as the negative logarithm of the base dissociation constant (Kb). A smaller pKb value indicates a stronger base.
- Primary (1°) Amine
- An amine formed when one hydrogen atom of ammonia is replaced by an alkyl (R) or aryl (Ar) group, resulting in a structure of the type RNH2 or ArNH2.
- Sandmeyer Reaction
- A reaction where a diazonium group is replaced by Cl–, Br–, or CN– by treating the diazonium salt with the corresponding copper(I) salt.
- Secondary (2°) Amine
- An amine formed when two hydrogen atoms of ammonia are replaced by alkyl or aryl groups, resulting in a structure of the type R-NHR’.
- Sulphonamide
- The product formed when primary or secondary amines react with benzenesulphonyl chloride (Hinsberg’s reagent).
- Tertiary (3°) Amine
- An amine formed when all three hydrogen atoms of ammonia are replaced by alkyl or aryl groups, resulting in a structure of the type R3N.




