Class 12 Chemistry Unit 6: Haloalkanes and Haloarenes – Mechanisms, Reactions & NCERT Notes
Unit 6: Haloalkanes and Haloarenes often serves as the entry point into the complex world of Class 12 Organic Chemistry. Many students struggle to connect the theoretical nature of the C-X bond with practical application in reaction mechanisms like SN1 and SN2. Instead of rote memorization, this study module focuses on the logic behind the reactions.
We break down the syllabus into manageable sections, covering everything from the specific conditions required for Sandmeyer’s reaction to the stereochemical implications of nucleophilic substitution. You will find visual comparisons for boiling point trends, a clear roadmap for solving organic conversions, and a dedicated breakdown of why haloarenes resist substitution. Use the interactive charts and reagent cheat sheets below to solidify your understanding and prepare for board exams.
Haloalkanes and Haloarenes
A complete study guide for Class XII (2025-26 Syllabus). Master organic reaction mechanisms, understand stereochemical principles, and apply logic to solve conversion problems.
1. Nature of C-X Bond
Halogen atoms are more electronegative than carbon. This creates a permanent dipole where carbon is slightly positive (electrophilic) and the halogen is slightly negative.
- Bond Polarity: C-F > C-Cl > C-Br > C-I
- Bond Length: C-F < C-Cl < C-Br < C-I
- Bond Enthalpy: Strongest in C-F, weakest in C-I.
Bond Parameters Visualization
Relative Bond Dissociation Enthalpy (Height)
2. Methods of Preparation
Synthesis Pathways to Haloalkanes (R-X)
From Alcohols (R-OH)
From Hydrocarbons
Halogen Exchange
3. Named Reaction Gallery
Sandmeyer’s Reaction
Preparation of Haloarenes from Diazonium Salts
Uses cuprous halide (Cu2X2) dissolved in corresponding halogen acid.
Gattermann Reaction
Modification of Sandmeyer
Uses Copper powder (Cu) instead of Cuprous halide.
Wurtz-Fittig Reaction
Coupling Alkyl + Aryl
Reaction in presence of dry ether to form Alkylbenzene.
4. Physical Properties Logic
BP Trend Boiling Points
Boiling points depend on van der Waals forces, which depend on molecular mass and surface area.
Visualizing Surface Area
Spherical molecules (highly branched) have less contact area with neighbors, leading to weaker attraction and lower boiling points.
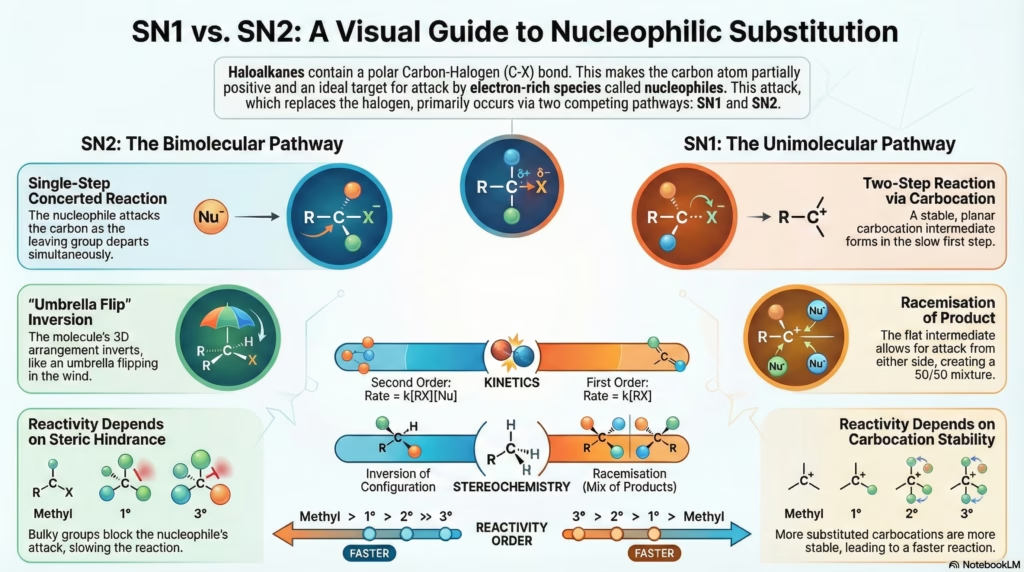
5. SN1 vs SN2 Mechanisms
| Feature | SN2 (Bimolecular) | SN1 (Unimolecular) |
|---|---|---|
| Kinetics | Second order. Rate = k[RX][Nu] | First order. Rate = k[RX] |
| Steps | Single Step (Concerted). | Two Steps (Ionization + Attack). |
| Intermediate | Transition State (Pentacoordinate). | Carbocation (Planar). |
| Stereochemistry | Inversion (Walden Inversion). “Umbrella” flips inside out. | Racemization. Attack from both sides of planar carbocation. |
| Order of Reactivity | Methyl > 1° > 2° > 3° (Steric hindrance controls rate) |
3° > 2° > 1° > Methyl (Carbocation stability controls rate) |
| Solvent | Polar Aprotic (Acetone, DMSO). | Polar Protic (Water, Alcohol). |
6. Reaction Energy Profiles
SN2 Profile (Single Step)
One smooth curve. Reactants go directly to Transition State (TS) and then to Products. No intermediate.
SN1 Profile (Two Steps)
Two distinct humps. The “valley” between them represents the stable Carbocation Intermediate.
7. Stereochemistry Essentials
Key Definitions
-
1
Chirality
Objects (or molecules) that are non-superimposable on their mirror image. Usually contains an asymmetric carbon (bonded to 4 different groups).
-
2
Enantiomers
Stereoisomers that are non-superimposable mirror images. They have identical physical properties but rotate plane-polarized light in opposite directions.
-
3
Racemic Mixture
A 1:1 mixture of two enantiomers. It has zero optical rotation because the rotation of one isomer cancels the other.
Chirality Concept: Hands are chiral.
Molecules with 1 chiral center behave just like hands.
8. Elimination Reactions (Dehydrohalogenation)
Reagent Trigger
Alcoholic KOH acts as a strong base, favoring elimination over substitution.
(Note: Aqueous KOH favors substitution to form Alcohol)
Zaitsev (Saytzeff) Rule
“In dehydrohalogenation reactions, the preferred product is that alkene which has the greater number of alkyl groups attached to the doubly bonded carbon atoms.”
Minor Product: Less substituted alkene
9. Master Reaction Map
The central hub of Alkyl Halide (R-X) Chemistry
10. Reagent Cheat Sheet
Quick reference for common reagents and ambident nucleophiles.
KOH (Potassium Hydroxide)
Cyanide Group (CN)
Nitrite Group (NO₂)
Reaction with Metals
11. Haloarenes Reactivity
Why are Haloarenes unreactive towards Nucleophilic Substitution?
Lone pairs on halogen delocalize into the benzene ring, creating partial double bond character in the C-X bond.
Carbon is sp2 hybridized (more electronegative) compared to sp3 in alkyl halides, holding the halogen tighter.
Deep Dive Forcing the Unreactive: Dow’s Process & Effect of NO₂
While typically unreactive, nucleophilic substitution can occur under drastic conditions or with electron-withdrawing groups.
623 K, 300 atm → Phenol
High temperature and pressure are required to break the strong C-Cl bond.
Presence of NO₂ at Ortho/Para positions increases reactivity.
Warm Water → Picric Acid
NO₂ stabilizes the carbanion intermediate.
Electrophilic Substitution Logic
Why are Halogens Ortho-Para directing despite being deactivating?
+M Effect (Resonance): The lone pair on Halogen donates electrons to the ring, increasing electron density specifically at Ortho and Para positions.
-I Effect (Inductive): The electronegative halogen pulls electrons, deactivating the overall ring.
Result: Reactivity is lower than benzene, but substitution happens at O/P positions.
12. Electrophilic Substitution Gallery
Chlorine is Ortho-Para directing but deactivating. Para product is usually Major due to symmetry.
Halogenation
Cl₂ / Anhyd. FeCl₃Nitration
HNO₃ / H₂SO₃Sulphonation
Conc. H₂SO₄ / HeatFriedel-Crafts
CH₃Cl / Anhyd. AlCl₃13. Conversion Roadmap
Use these standard pathways to solve conversion problems.
Ascent Increasing Carbon Chain Length
Transform Common Transformations
14. Strategic Problem Solving
How to solve “Identify A, B, C”?
Look for the End Product: If the end product is an acid, the precursor was likely a Nitrile or Alcohol.
Count the Carbons: If Carbon count increases, look for KCN or Grignard. If it stays same, standard substitution.
Check for Isomers: If the question mentions “optical activity” or “racemic mixture”, think SN1 mechanism.
Example Logic Flow
15. Common Exam Pitfalls
⚠️ Dont’s
- Don’t forget to account for Hydride/Methyl shifts in SN1 carbocations.
- Don’t use aqueous KOH if you want an Alkene (Use Alcoholic!).
- Don’t assume Chlorobenzene undergoes substitution easily.
✅ Do’s
- Do write “Major” and “Minor” products in Electrophilic substitution.
- Do mention “Inversion of configuration” for SN2 questions.
- Do check if the halide is vinylic or aryl (Low Reactivity).
16. Polyhalogen Compounds
Chloroform (CHCl3)
Used as a solvent. Slowly oxidizes in air/light to form poisonous Phosgene gas.
Iodoform (CHI3)
Previously used as an antiseptic. The antiseptic action is due to the liberation of free Iodine, not the compound itself.
Freons (CFCs)
Stable, unreactive, non-toxic gases used in aerosol propellants and cooling. Major cause of Ozone Layer Depletion.
17. Conceptual Deep Dive
AgCN is predominantly covalent. The silver atom blocks the carbon atom, leaving only the Nitrogen lone pair available for nucleophilic attack, resulting in an Isocyanide (R-NC).
18. Interactive Q&A Practice
Why are haloalkanes insoluble in water despite the C-X bond being polar?
Why is sulphuric acid (H2SO4) not used during the reaction of alcohols with KI?
Which compound reacts faster in SN1 reaction: 2-Bromo-2-methylpropane or 2-bromopropane? Why?
Explain why Grignard reagents should be prepared under anhydrous conditions.
Identify the product formed when Chlorobenzene is treated with Sodium in the presence of dry ether.
2C6H5Cl + 2Na → C6H5-C6H5 + 2NaCl
Environmental Awareness
While Polyhalogen compounds like Freons were industrial milestones, their stability led to Ozone layer depletion. Modern chemistry prioritizes environmental safety alongside utility.