Class 12 Coordination Compounds (Unit 5): Notes, CFT Visualizer & Practice Questions
Transition metals possess a unique ability to bind with neutral molecules or anions to form complex structures known as coordination compounds. Unlike simple double salts that break down completely in water, these entities retain their identity and distinct physical properties.
This guide breaks down the core principles governing these structures, starting from Alfred Werner’s early postulates to modern bonding theories. Students will examine how Crystal Field Theory explains the vivid colours seen in transition metal complexes and how Valence Bond Theory predicts their geometry and magnetic behavior.
The included interactive modules and timed assessments provide a practical way to master IUPAC nomenclature and stereoisomerism for the upcoming board exams.
Rezyo.in
Class XII Unit 5: Coordination Compounds
Coordination Compounds: The Chemistry of Colour
Beyond simple salts lies the complex world of Coordination Chemistry. This unit bridges ionic bonding and molecular structure, explaining how transition metals bind with ligands to form colourful, magnetic, and biologically vital compounds like Haemoglobin and Cisplatin.
Knowledge Check
Pop-out quiz with timer to test your grasp on VBT, CFT, and Nomenclature.
Coordination Mastery Challenge
Ready to test your understanding of Ligands, Isomerism, and Bonding Theories? You have 35 questions. The clock starts when you click below.
Fundamental Terminology
Coordination Entity
The central metal atom bonded to a fixed number of ions or molecules.
Ligands
Lewis bases donating electron pairs to the metal. Classified by denticity.
Concept: Ambidentate Ligands
Ligands with two potential donor atoms but which bond through only one at a time.
Examples:
NO2–: Bond via N (Nitro) or O (Nitrito).
SCN–: Bond via S (Thiocyanato) or N (Isothiocyanato).
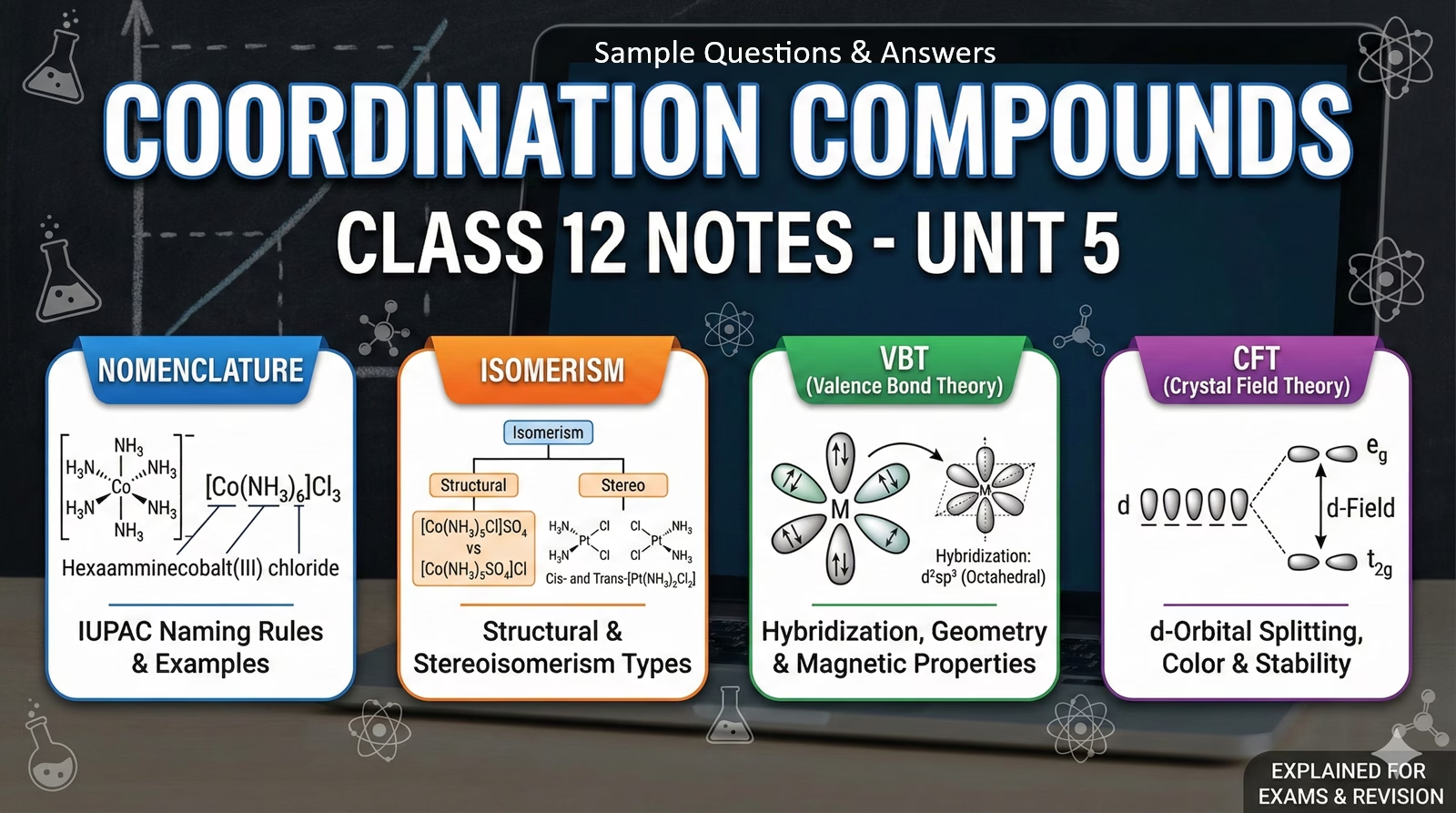
IUPAC Nomenclature Rules
- Order: Cation named first, then Anion.
- Alphabetical: Ligands are named alphabetically before the metal.
- Prefixes: Use di-, tri- for simple ligands; bis-, tris- for complex ligands (like ‘en’).
- Metal Ending: If complex is anionic, metal ends in -ate (e.g., Ferrate).
| Formula | Analysis | IUPAC Name |
|---|---|---|
| [Co(NH3)6]Cl3 | Cationic complex. Co(III). | Hexaamminecobalt(III) chloride |
| K3[Fe(C2O4)3] | Anionic complex. Fe(III). | Potassium trioxalatoferrate(III) |
| [Ni(CO)4] | Neutral. Ni(0). | Tetracarbonylnickel(0) |
Valence Bond Theory (VBT)
Predicts geometry based on hybridization of metal orbitals.
CFT: Crystal Field Splitting
Splitting of d-orbitals in Octahedral Field
Isomerism
Structural Isomerism
- Linkage: Ambidentate ligands (NO2 vs ONO).
- Solvate: Water inside/outside sphere ([Cr(H2O)6]Cl3 vs [Cr(H2O)5Cl]Cl2.H2O).
- Ionization: Exchange of counter ions (SO4 vs Br).
- Coordination: Exchange between cationic/anionic complexes.
Stereoisomerism
- Geometrical: Cis (Adjacent) vs Trans (Opposite). Common in square planar and octahedral.
- Optical: Enantiomers (Non-superimposable mirror images). Common in octahedral complexes with chelating ligands (e.g., [Co(en)3]3+).
Formula Cheat Sheet
n = unpaired electrons
Plus Pairing Energy (P) if needed
Tetrahedral vs Octahedral
Conceptual Doubts (FAQ)
Q: Why are Zn2+ complexes colourless?
Zn2+ has a 3d10 configuration (completely filled d-orbitals). There are no empty orbitals for electrons to transition into (no d-d transition possible), hence no visible light is absorbed.
Q: What is the Chelate Effect?
Complexes containing chelating (ring-forming) ligands like ‘en’ or EDTA are significantly more stable than those with monodentate ligands. This is an entropy-driven process.
Q: How does the Spectrochemical Series work?
It arranges ligands by field strength. Weak ligands (Halogens) cause small splitting (High Spin). Strong ligands (CN, CO) cause large splitting (Low Spin/Pairing).
I– < F– < H2O < NH3 < CN– < CO
Q: Why is CO a stronger ligand than NH3?
CO engages in Synergic Bonding. It acts as a sigma donor (C to Metal) and a pi acceptor (Metal to C), strengthening the bond via back-donation.
Chapter Summary
We have explored the architecture of coordination compounds. From Werner’s primary valencies to the orbital splitting of CFT, you now have the tools to predict geometry, magnetic behavior, and colour. Remember: Geometry depends on Hybridization (VBT), but Colour depends on Splitting (CFT).