Coordination Compounds Class 12 Notes Unit 5: Nomenclature, Isomerism, VBT & CFT Explained
Transition metals possess the unique ability to form complex structures known as coordination compounds. Unlike simple double salts such as Carnallite which dissociate completely in water, coordination entities retain their identity due to strong metal-ligand coordinate bonds.
This unit covers the fundamental principles governing these structures, starting with Werner’s postulates and moving through IUPAC nomenclature rules.
Students will examine the geometry and magnetic properties of complexes using Valence Bond Theory (VBT) and understand colour mechanisms via Crystal Field Theory (CFT).
Coordination Compounds
Transition metals form structures known as coordination compounds. Unlike double salts which dissociate completely in water (like Carnallite), coordination compounds retain their identity. The central metal ion binds to ligands via coordinate bonds.
Werner’s Theory
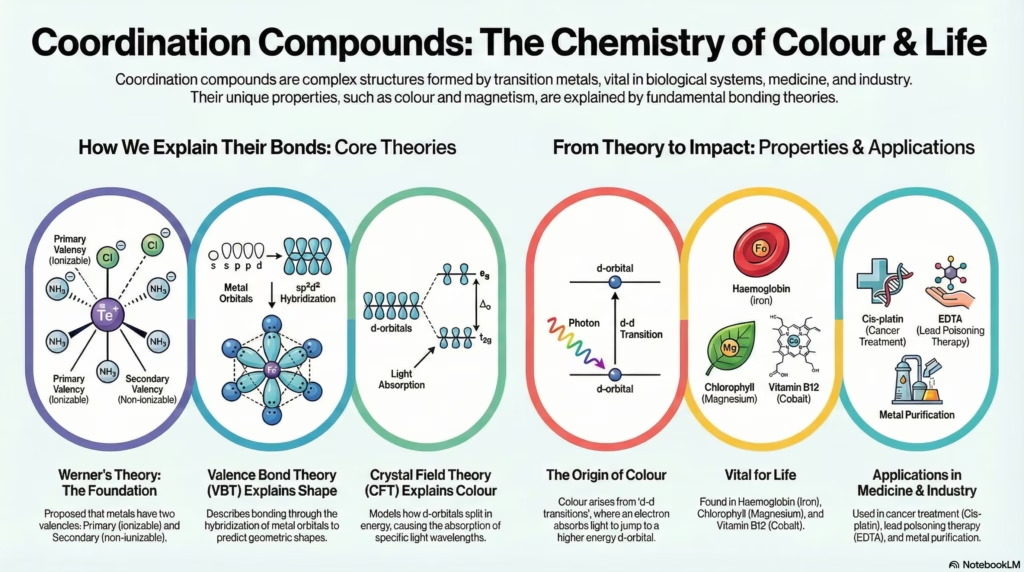
Alfred Werner proposed that metals in coordination compounds exhibit two types of valencies. This theory laid the foundation for modern coordination chemistry.
1. Primary Valency
- Corresponds to the Oxidation State.
- Ionizable (satisfied by anions).
- Represented by dotted lines.
- Example: In CoCl3·6NH3, 3 Cl– ions satisfy primary valency.
2. Secondary Valency
- Corresponds to the Coordination Number.
- Non-ionizable (satisfied by neutral molecules or anions).
- Represented by solid lines.
- Example: In CoCl3·6NH3, 6 NH3 satisfy secondary valency.
[Co(NH3)6]Cl3 + 3AgNO3 → 3AgCl (ppt)
Basics & Terminology
The Coordination Sphere
Click “Re-Draw” to visualize the structure of [Co(NH3)6]Cl3
Coordination Entity
The central metal atom or ion bonded to a fixed number of ions or molecules. In [CoCl3(NH3)3], the entity is the Cobalt ion surrounded by three chloride ions and three ammonia molecules.
Ligands
Ions or molecules bound to the central atom. They act as Lewis bases donating electron pairs.
| Type | Description | Examples |
|---|---|---|
| Monodentate | One donor atom | H2O, NH3, Cl– |
| Bidentate | Two donor atoms | en (H2NCH2CH2NH2), C2O42- |
| Polydentate | Multiple donor atoms | EDTA4- (Hexadentate) |
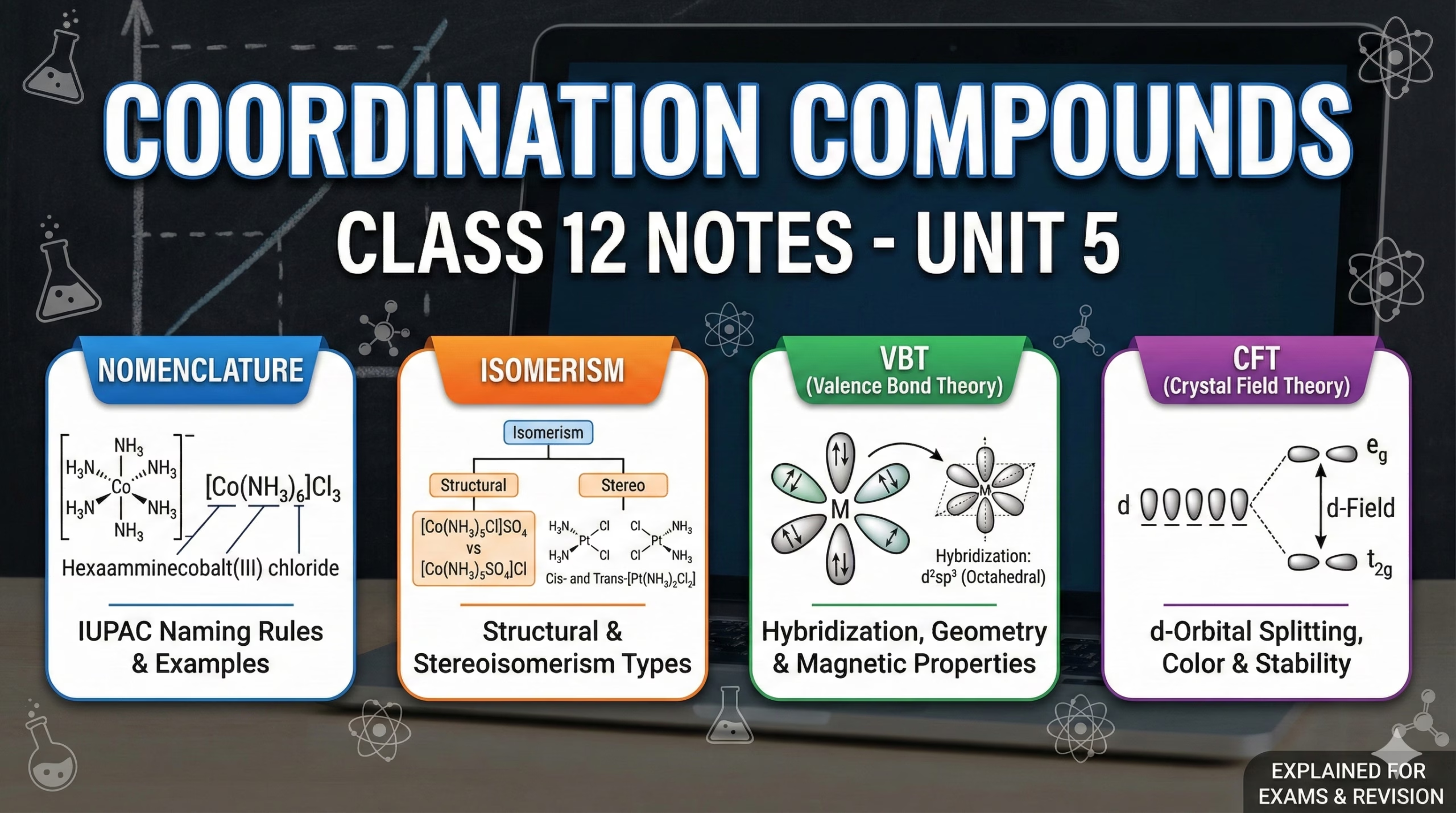
IUPAC Nomenclature
Cation is named first. Anion second. Within the complex entity, ligands are named alphabetically before the metal.
Anionic ligands end in -o (e.g., Chlorido). Neutral ligands keep names (exceptions: Aqua, Ammine, Carbonyl).
If the complex is anionic, the metal name ends in -ate (Ferrate, Argentate). Oxidation state follows in parentheses (II).
Common Examples
| Formula | Thinking Process | IUPAC Name |
|---|---|---|
| [Co(NH3)6]Cl3 | Cationic complex. Ligand: Ammine (x6). Metal: Cobalt. Ox. State: +3. | Hexaamminecobalt(III) chloride |
| K3[Fe(C2O4)3] | Anionic complex. Counter ion: Potassium. Ligand: Oxalato. Metal: Ferrate. | Potassium trioxalatoferrate(III) |
| [Pt(NH3)2Cl(NO2)] | Neutral. Alphabetic: Ammine, Chlorido, Nitrito-N. | Diamminechloridonitrito-N-platinum(II) |
Isomerism
Structural Isomerism
Linkage Isomerism
Occurs with ambidentate ligands.
Example: [Co(NH3)5(NO2)]Cl2 (Yellow) vs [Co(NH3)5(ONO)]Cl2 (Red)
Solvate Isomerism
Water molecules exchange between coordination sphere and outside lattice.
Example: [Cr(H2O)6]Cl3 (Violet) vs [Cr(H2O)5Cl]Cl2.H2O (Grey-Green)
Ionization Isomerism
Exchange of counter ion and ligand.
Example: [Co(NH3)5SO4]Br vs [Co(NH3)5Br]SO4
Stereoisomerism
Geometrical: Cis vs Trans
Geometric: Different spatial arrangement of ligands. Common in square planar and octahedral complexes.
Optical: Non-superimposable mirror images (Enantiomers). Occurs in octahedral complexes with chelating ligands like [Co(en)3]3+.
Theories of Bonding
Valence Bond Theory (VBT)
- Focuses on orbital hybridization (sp3, dsp2, etc.).
- Explains geometry and magnetic properties.
- Distinguishes Inner Orbital (d2sp3) vs Outer Orbital (sp3d2) complexes.
- Does not explain colour or spectra well.
Crystal Field Theory (CFT)
- Electrostatic model. Ligands are point charges.
- Explains colour via d-d transitions.
- Describes splitting of d-orbitals into t2g and eg sets.
- Introduces Crystal Field Stabilization Energy (CFSE).
Crystal Field Splitting (Octahedral)
Watch how d-orbitals split in the presence of ligands. Electrons jump from lower (t2g) to higher (eg) energy levels by absorbing light.
Octahedral vs Tetrahedral Splitting
Ligands approach along the axes. Orbitals on axes (dx2-y2, dz2) are repelled more.
Result: eg (high) & t2g (low)
Ligands approach between axes. Splitting is inverted and smaller.
Result: t2 (high) & e (low)
Δt = (4/9) Δo
Colour in Coordination Compounds
Complementary Colour Wheel
The observed colour is the complement of the absorbed colour.
Why do they exhibit colour?
It arises from d-d transitions. When white light falls on the complex, electrons in lower d-orbitals absorb specific wavelengths to jump to higher d-orbitals. The remaining light is transmitted.
Example: [Ti(H2O)6]3+
- Absorbs: Yellow-Green light.
- Transmits: Violet (its complement).
- If ligands are removed (e.g., heating), the splitting disappears, and the substance becomes colourless.
Carbonyls & Magnetism
Metal Carbonyls & Synergic Bonding
Organometallic compounds where Carbon Monoxide (CO) acts as a ligand are called Metal Carbonyls (e.g., [Ni(CO)4]). The stability of these complexes arises from the Synergic Effect.
- 1 Sigma (σ) Bond: Lone pair from Carbon donates to the empty d-orbital of the Metal.
- 2 Pi (π) Back-Bond: Filled d-orbital of Metal donates electrons back into the empty anti-bonding π* orbital of Carbon.
Magnetic Properties
The magnetic moment depends on the number of unpaired electrons (n).
Formula: μ = √n(n+2) BM (Bohr Magneton)
Diamagnetic: All electrons are paired.
Spin Only Magnetic Moment Calculator
Applications
Medicine
- Cis-platin: [PtCl2(NH3)2] effectively inhibits the growth of tumors (Cancer treatment).
- EDTA: Used in treating lead poisoning.
Biological Systems
- Haemoglobin: Red pigment of blood, a complex of Iron (Fe).
- Chlorophyll: Green pigment in plants, a complex of Magnesium (Mg).
- Vitamin B12: Cyanocobalamin, a complex of Cobalt (Co).
Extraction & Analysis
- Gold/Silver Extraction: Uses cyanide complexes [M(CN)2]–.
- Water Hardness: Estimated using EDTA titration (Ca2+ and Mg2+ complexes).
- Mond Process: Nickel purification via [Ni(CO)4].
Stability of Coordination Compounds
The stability in solution refers to the equilibrium between the complex and its constituent ions.
Stability Constant (β): Higher values indicate greater stability.
M + 4L ⇌ ML4
Chelate Effect: Complexes formed by chelating ligands (ring-forming like ‘en’) are more stable than those formed by monodentate ligands.
Q&A and FAQ
Practice Problems
1. Why is [Ti(H2O)6]3+ violet?
2. Double Salt vs Complex?
3. Geometry of [Ni(CN)4]2-?
4. Oxidation state of Co in [Co(en)3]3+?
Frequently Asked Questions
Why are Zn2+ complexes colourless?
How do I distinguish between Inner and Outer orbital complexes?
- Inner Orbital: Uses (n-1)d orbitals (e.g., d2sp3). Usually formed with strong field ligands (Low spin).
- Outer Orbital: Uses nd orbitals (e.g., sp3d2). Usually formed with weak field ligands (High spin).
What is the Spectrochemical Series?
I– < Br– < Cl– < F– < OH– < H2O < NH3 < en < CN– < CO CO causes maximum splitting (strongest), while I– causes minimum (weakest).
Chapter Summary
Unit 5 Complete • Class XII Chemistry