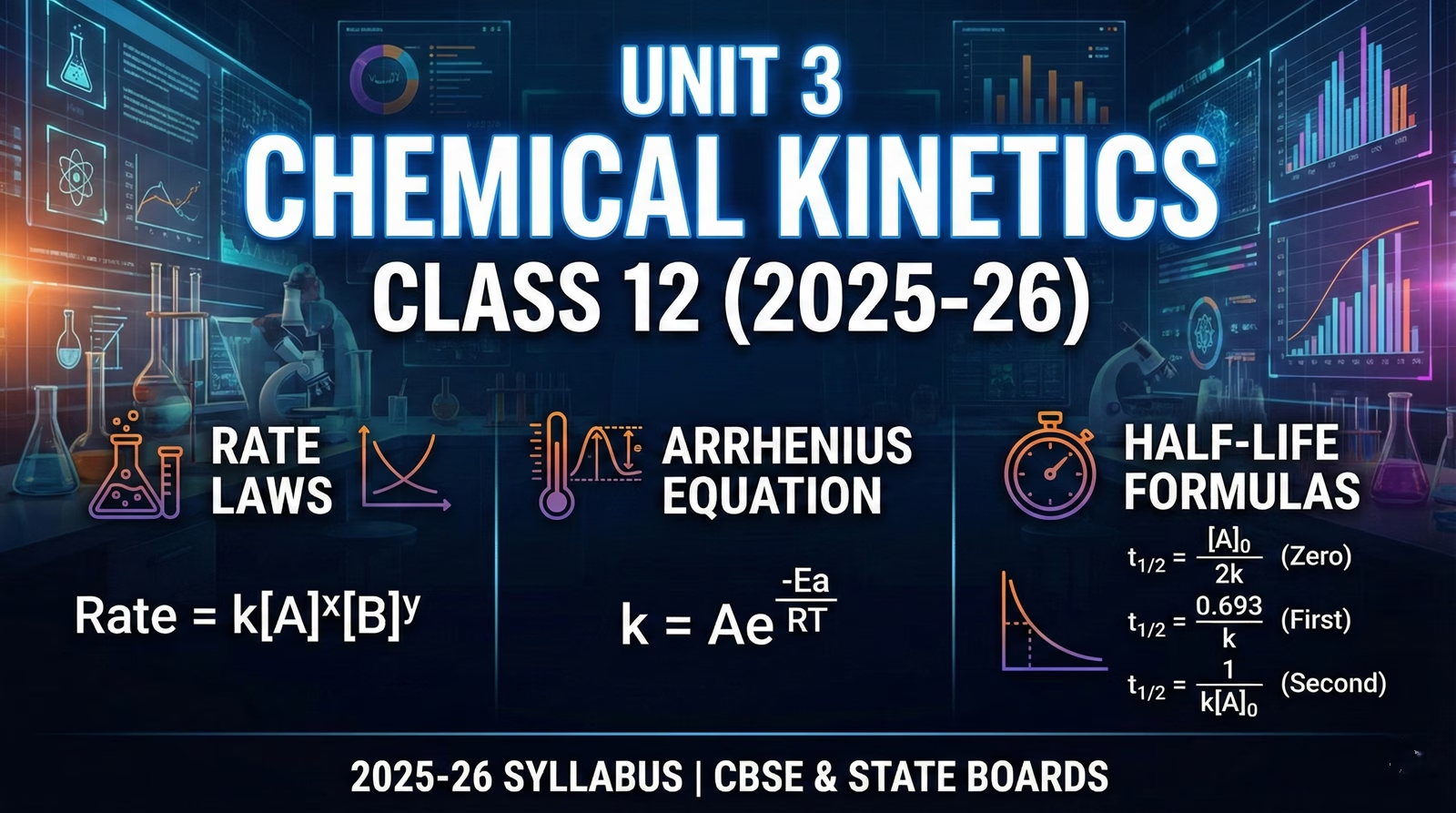
Unit 3 Chemical Kinetics Class 12 (2025-26): Rate Laws, Arrhenius Equation & Half-Life Formulas
Thermodynamics predicts if a reaction occurs, but Chemical Kinetics determines the speed. For Class XII students tackling the 2025-26 syllabus, mastering the “time” variable in chemistry is required for solving numerical problems and interpreting rate graphs correctly.
Try our Practice Questions & Answers
This guide breaks down the mathematics of reaction rates, comparing Average and Instantaneous speeds while distinguishing experimental Order from theoretical Molecularity. From deriving Integrated Rate Equations for Zero and First Order reactions to calculating Activation Energy using the Arrhenius formula, these sections provide the specific tools needed for board exams.
Explore how concentration, temperature, and catalysts dictate reaction velocity through interactive visualizers designed to clarify the mechanics behind the math.
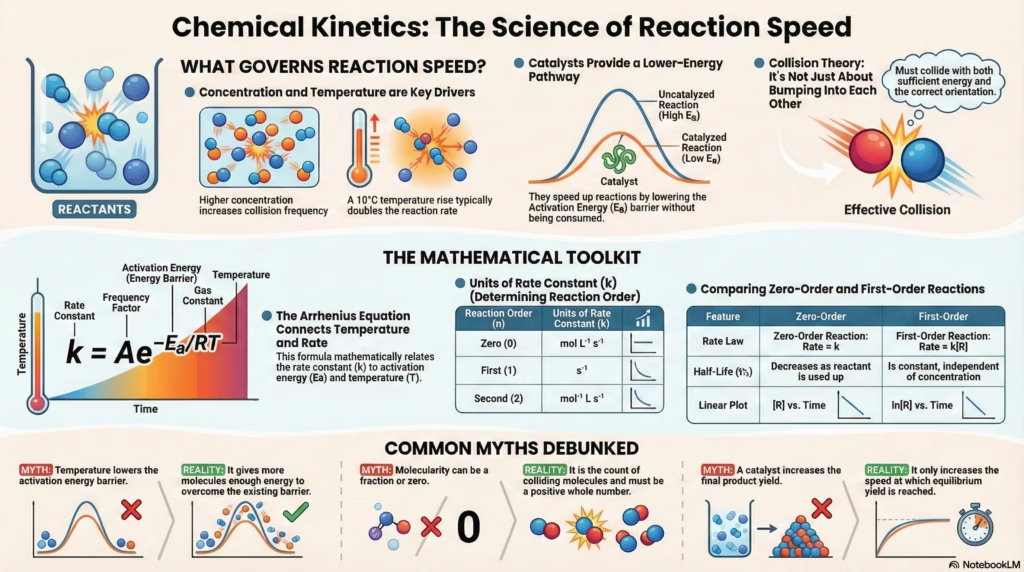
Unit 3: Chemical Kinetics
The ultimate guide to reaction rates, integrated rate laws, and collision theory. Merging conceptual depth with interactive visual learning.
1 Defining Reaction Rate
Rate defines speed. In chemistry, it is the change in concentration of a reactant or product per unit time.
Average Rate
Measured over a finite time interval.
Instantaneous Rate
Measured at a specific moment (\(\Delta t \rightarrow 0\)).
Stoichiometric Normalization
2 Factors Affecting Rate
Concentration
Higher concentration increases collision frequency.
Rate ∝ [Reactant]ⁿ
Temperature
For every 10°C rise, rate typically doubles (Temperature Coefficient \(\mu \approx 2\)).
Catalyst
Provides an alternate pathway with lower Activation Energy (\(E_a\)).
3 Module: Collision Theory & Orientation
Collision frequency (\(Z\)) isn’t enough. Molecules must hit with proper Orientation and Energy.
The Steric Factor (\(P\)) accounts for this probability.
4 Reaction Mechanisms
Elementary vs Complex
Elementary: Occurs in a single step. Order = Molecularity.
Complex: Occurs in multiple steps. The overall rate is controlled by the slowest step.
RDS: Rate Determining Step
Just as the narrowest part of a funnel limits flow, the slowest step limits reaction speed.
Example: \( 2NO + O_2 \rightarrow 2NO_2 \)
- Step 1 \( NO + NO \rightleftharpoons N_2O_2 \) (Fast)
- Step 2 \( N_2O_2 + O_2 \rightarrow 2NO_2 \) (Slow/RDS)
The rate law is derived from Step 2, substituting the intermediate \(N_2O_2\) using the equilibrium constant from Step 1.
5 The Units of Rate Constant (k)
The unit of \(k\) depends on the order of the reaction (\(n\)). This is a key identifier in problems.
General Formula
| Order (n) | Formula Substitution | Final Unit |
|---|---|---|
| Zero (0) | (mol/L)1-0 s-1 | mol L-1 s-1 |
| First (1) | (mol/L)1-1 s-1 | s-1 |
| Second (2) | (mol/L)1-2 s-1 | mol-1 L s-1 |
6 Module: Half-Life Dynamics
How does half-life change as the reaction proceeds?
Zero Order: Half-life decreases as concentration drops.
First Order: Half-life remains constant regardless of concentration.
Select a reaction order above.
7 Integrated Rate Equations
Zero Order
First Order
8 Module: Graphical Fingerprints
To determine the order experimentally, chemists look for linear plots. Toggle below to see which plot yields a straight line for each order.
9 Numerical Master Key
The Two-Temperature Formula
Used when rate constants (\(k_1, k_2\)) are given at two different temperatures (\(T_1, T_2\)) to find \(E_a\).
* Note: \(T\) must be in Kelvin.
* \(R = 8.314\ J\ K^{-1}\ mol^{-1}\).
Problem Solving Steps
- Convert all Temperatures to Kelvin (\(+273\)).
- Identify \(k_1\) (lower temp) and \(k_2\) (higher temp).
- Calculate \(\log(k_2/k_1)\). If rate doubles, this is \(\log 2 = 0.301\).
- Rearrange formula to solve for \(E_a\).
- Final unit of \(E_a\) is usually \(J\ mol^{-1}\) or \(kJ\ mol^{-1}\).
10 Arrhenius Equation & Collision Theory
When plotting \(\ln k\) vs \(1/T\):
Slope: \(-E_a/R\)
Intercept: \(\ln A\)
Effective Collisions
- Energy Barrier: Kinetic energy \(\ge E_a\).
- Orientation Barrier: Proper geometric alignment.
11 Catalyst & Energy
A catalyst provides an alternate pathway with a lower activation energy (\(E_a’\)).
Concept Buster: Myth vs Reality
Tap a card to reveal the scientific truth.
Deep Dive FAQ
It’s not because collision frequency (\(Z\)) doubles; \(Z\) increases only slightly ($\approx 3\%$). The main reason is the exponential increase in the fraction of molecules having energy greater than \(E_a\). This “effective” population nearly doubles.
Molecularity is defined only for elementary (single-step) reactions. A complex reaction proceeds in multiple steps. We can define molecularity for each step, but not for the overall reaction. Order, however, is an experimental value for the overall reaction.
Generally, no. Molecules need excess energy to break bonds. However, in rare barrier-less reactions (like radical combinations), \(E_a \approx 0\). A theoretical “negative” \(E_a\) usually implies a complex mechanism where an intermediate equilibrium is sensitive to temperature.
Learning Outcomes & Conclusion
You have mastered:
- Reaction Dynamics: How concentration, temperature, and orientation dictate speed.
- Mechanism Analysis: Identifying the Rate Determining Step (RDS).
- Integrated Laws: Calculating \(k\) and \(t_{1/2}\) for Zero/First order.
- Arrhenius Logic: Connecting Temperature and Activation Energy graphically.
Exam Strategy: Spot the Order
In exams, if the order isn’t given, look at the units of \(k\):
- \(s^{-1}\) → First Order
- \(mol\ L^{-1}\ s^{-1}\) → Zero Order
- \(mol^{-1}\ L\ s^{-1}\) → Second Order